1318
Distortion-Free Diffusion-Relaxometry Imaging with Self-navigated Cartesian-based Echo-Planar Time Resolved Acquisition (cEPTI)1Department of Radiology, Stanford University, Stanford, CA, United States, 2Department of Electrical Engineering, Stanford University, Stanford, CA, United States, 3Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 4Department of Electrical Engineering and Computer Science, MIT, Cambridge, MA, United States
Synopsis
Recently, there has been a growing interest in developing distortion-free EPI acquisitions for high-fidelity diffusion, relaxometry and/or functional MRI. Point spread function (PSF)-based techniques have been proposed for distortion-free diffusion imaging. Another technique, echo-planar time resolved imaging (EPTI), has been demonstrated for distortion-free relaxometry with an EPI readout. Additionally, a combination of PROPELLER and EPTI has been reported for motion-robust simultaneous diffusion and relaxometry imaging. In this study, we develop a self-navigated Cartesian-based EPTI (cEPTI) acquisition for distortion-free diffusion-relaxometry imaging. In vivo human brain data demonstrate that high-quality distortion-free diffusion and relaxometry images can be acquired with the proposed cEPTI.
Purpose
Distortion-free multi-shot EPI acquisitions represent a compelling approach to high-fidelity diffusion, relaxometry and/or functional MRI. Point spread function (PSF)-based techniques have been proposed for distortion-free diffusion imaging 1,2. A similar technique, echo-planar time resolved imaging (EPTI), has also been demonstrated for distortion-free relaxometry imaging with an EPI readout 3. Additionally, a combination of PROPELLER and EPTI (PEPTIDE) has been reported for motion-robust simultaneous diffusion and relaxometry imaging 4,5. In this study, we aim to develop a self-navigated Cartesian-based EPTI (cEPTI) acquisition for distortion-free diffusion-relaxometry imaging.Methods
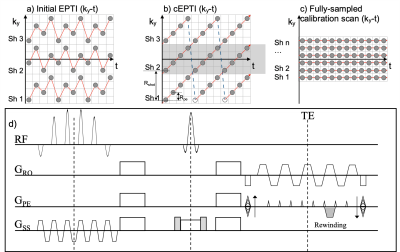
Sequence Design As with other multi-shot EPI acquisitions, inter-shot phase variations represent the main challenge associated with incorporating EPTI into diffusion imaging. In conventional multi-shot diffusion imaging, this can be solved by using an internal or external navigator image 6-8. With the conventional EPTI implementation 3, not all the shots traverse the center of k-space (Fig. 1a), so external navigators would be needed for inter-shot phase corrections. Here, we first design a self-navigated Cartesian EPTI sequence (Fig. 1b). Meanwhile, in our cEPTI, data is uniformly sampled at each echo time, which maintains the ability to acquire quantitative relaxometry data as in the conventional EPTI 3.The self-navigated cEPTI is implemented by introducing a rewinding gradient when ky reaches ky, max during the readout (Fig. 1d). With the rewinding gradient, the sampling trajectory will be reversed back to -ky, max and then moves towards ky, max again until the end of the readout. The area of the rewinding gradient is:
Arewind=(2π)/(γΔy)
where γ is the gyromagnetic ratio, Δy is the spatial resolution. There are two highlights of the rewinding gradient. First, the reversing from ky, max to -ky, max occurs at different echo times for different shots (blue dashed lines in Fig. 1b). Therefore, the time to add the rewinding gradient also changes over different shots. Second, when adding the rewinding gradient, both kx and ky locations will change simultaneously, which indicates that the corresponding sampled data do not lie on a single ky line. In this study, we simply discard the sampling points during the rewinding gradient and denote them with a hollow circle in Fig. 1b.
Data Acquisition Data were acquired on a GE 3.0T Premier MRI scanner, using a 48-channel head coil. The imaging parameters were: FOV=220×220 mm2, in-plane resolution=1.0×1.0 mm2, slice thickness=3 mm, 20 slices, echo spacing time=1.0 ms, TR=2.8 s, 30 diffusion encoding directions with b=1000 s/mm2. Two under-sampling factors are defined in cEPTI (Fig. 1b): Rpe is the ky step in each shot to adjust the sampling locations and Rshot is the step of the starting ky location between two adjacent shots for acceleration. Fig. 1b shows an example when Rpe=1 and Rshot=4, 3 shots are used to sample the ky-t space (Nshot=3) without applying partial Fourier (PF=1). The actual parameters of the cEPTI acquisition in this study were: Rpe=4 and Rshot=22, Nshot=8 and PF=0.8. CAIPI sampling 9,10 was used in the ky-t domain. Forty-eight echoes (Necho=48) were acquired as a proof-of-concept, with the TE of the spin echo located at the 17th echo (TESE=63 ms). The total scan time was 11min40sec.
A fully sampled cEPTI (Rpe=0, Rshot=1) was acquired as the time-resolved 2D calibration scan (kx-ky-t) (Fig. 1c). Rpe=0 means that in each shot, all echoes were sampled at the same ky location. The time-resolved 2D calibration scan contained the coil sensitivity and the field susceptibility induced phase at each echo time.
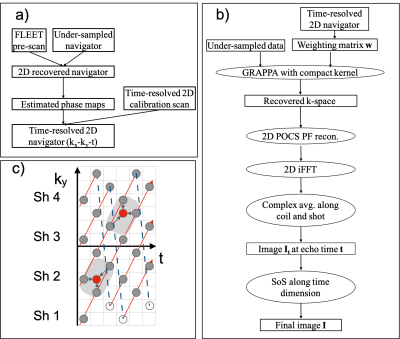
Reconstruction The preprocessing of the navigator is described in Fig. 2a. Each shot was recovered using GRAPPA 11 and the central k-space of each shot was extracted as the navigator. Then the phase of each shot was calculated by multiplying the diffusion navigators with the conjugate of the b0 navigators from the same shot. In this way, we removed both the coil sensitivity phase and field susceptibility induced phase from the diffusion navigator and only kept the motion-induced phase. The phase was duplicated Necho times and added to the time-resolved 2D calibration data (kx-ky-t) at each echo time, synthesizing a time-resolved navigator with matched inter-shot phase variation, coil sensitivity map and field susceptibility induced phase as the diffusion cEPTI data at each echo time.
GRAPPA with compact kernel (GRAPPA-CK) 12 was extended for the inter-shot phase correction in diffusion cEPTI, with the weighting matrix estimated from the synthesized time-resolved 2D navigator (kx-ky-t) (Fig. 2b). An example of the interpolation process is shown in Fig. 2c.
Results
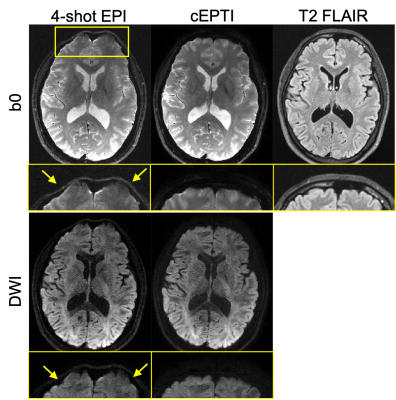
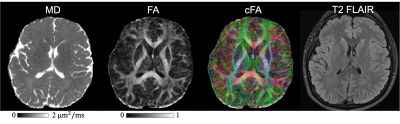
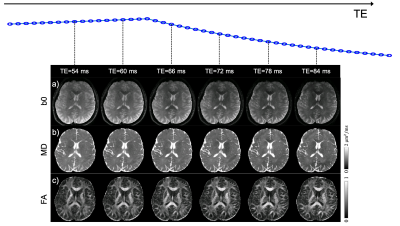
Fig. 3a shows a comparison of the diffusion images using the proposed cEPTI and the conventional 4-shot EPI acquisition, with a T2 FLAIR image as distortion-free reference. Residual distortion artifacts can still be observed in the 4-shot EPI results but are absent in the cEPTI results.Fig. 4 displays the MD, FA and color-coded FA maps from the combined images over all the echoes. Fig. 5 shows the b0 image, MD and FA maps at six representative echo times, demonstrating the potential of cEPTI for simultaneous diffusion and relaxometry imaging.
Discussion & Conclusion
The development of a self-navigated Cartesian-based EPTI acquisition method for distortion-free diffusion-relaxometry MRI represents a promising acquisition strategy for a broad range of clinical and research applications including many microstructure imaging techniques.Acknowledgements
This study is funded by GE Healthcare and the Focused Ultrasound Foundation.References
1. Dong Z, Wang F, Reese TG, et al. Tilted-CAIPI for highly accelerated distortion-free EPI with point spread function (PSF) encoding. Magn Reson Med 2019;81:377-392.
2. In M-H, Posnansky O, Speck O. High-resolution distortion-free diffusion imaging using hybrid spin-warp and echo-planar PSF-encoding approach. Neuroimage 2017;148:20-30.
3. Wang F, Dong Z, Reese TG, et al. Echo planar time-resolved imaging (EPTI). Magn Reson Med 2019;81:3599-3615.
4. Fair M, Liao C, Kim D, et al. Diffusion-PEPTIDE: rapid distortion-free diffusion-relaxometry imaging. In Proceedings of the 28th Annual Meeting of ISMRM, Virtual Meeting, 2020. Abstract 0953.
5. Fair MJ, Wang F, Dong Z, Reese TG, Setsompop K. Propeller echo-planar time-resolved imaging with dynamic encoding (PEPTIDE). Magn Reson Med 2020;83:2124-2137.
6. Chen NK, Guidon A, Chang HC, Song AW. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). Neuroimage 2013;72:41-47.
7. Jeong HK, Gore JC, Anderson AW. High-resolution human diffusion tensor imaging using 2-D navigated multishot SENSE EPI at 7 T. Magn Reson Med 2013;69:793-802.
8. Miller KL, Pauly JM. Nonlinear phase correction for navigated diffusion imaging. Magn Reson Med 2003;50:343-353.
9. Breuer FA, Blaimer M, Heidemann RM, et al. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magn Reson Med 2005;53:684-691.
10. Setsompop K, Gagoski BA, Polimeni JR, et al. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med 2012;67:1210-1224.
11. Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magnetic Resonance in Medicine 2002;47:1202-1210.
12. Ma X, Zhang Z, Dai E, Guo H. Improved multi-shot diffusion imaging using GRAPPA with a compact kernel. Neuroimage 2016;138:88-99.
Figures