1316
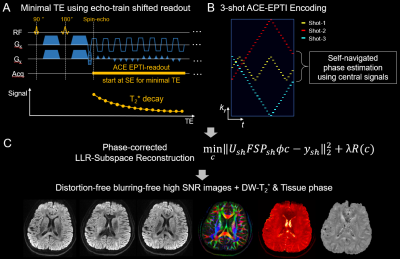
ACcelerated Echo-train shifted EPTI (ACE-EPTI) for fast distortion-blurring-free high-resolution diffusion imaging with minimal echo time1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Electrical Engineering and Computer Science, MIT, Cambridge, MA, United States, 3Harvard-MIT Health Sciences and Technology, MIT, Cambridge, MA, United States, 4Department of Radiology, Stanford University, Stanford, CA, United States, 5Department of Electrical Engineering, Stanford University, Stanford, CA, United States
Synopsis
ACcelerated Echo-train shifted EPTI (ACE-EPTI) is proposed for fast and high-fidelity dMRI and diffusion-relaxometry. Distortion- and blurring-free imaging at high resolution is achieved using the time-resolved imaging approach, which recovers sharp multi-contrast images at each echotime across a continuous readout window. A new spatiotemporal encoding is proposed for rapid acquisition, requiring only 3 shots for submillimeter imaging with the capability of self-navigated physiological phase correction. An echo-training shifting acquisition is also incorporated into ACE-EPTI to enable shortest possible TE, which provides 30-40% higher SNR-efficiency over single-shot EPI. High-quality diffusion images and diffusion-weighted quantitative maps acquired by ACE-EPTI are demonstrated in-vivo.
Introduction
EPI is the most commonly used technique for diffusion imaging. However, distortion and blurring artifacts of EPI limit its ability to achieve high spatial resolution and study detailed structures in vivo. Many efforts have been made to improve EPI, including multi-shot EPI1-3 to reduce distortion, blip-up and down acquisition to correct for distortion4,5, and point-spread-function-encoding (PSF-EPI)6,7 to eliminate both distortion and blurring. To accelerate PSF-EPI, which usually requires >30 EPI-shots per diffusion direction, the tilted-CAIPI method8 has been proposed to enable 0.8-mm imaging in 8-shots. Recently, another distortion- and blurring-free technique has been developed, termed echo-planar time-resolved imaging (EPTI)9, which provides time-resolved multi-contrast imaging capability for extraction of relaxometry information. EPTI has recently been combined with PROPELLER readout10,11 for motion-robust diffusion-relaxometry imaging, however, >10 shots are required for submillimeter imaging.In this work, we present ACcelerated Echo-train shifted EPTI (ACE-EPTI), which achieves distortion- and blurring-free high-quality diffusion and diffusion-relaxometry imaging at submillimeter resolution in just 3 shots. This is made possible through a novel k-t acquisition design that also enables self-navigation of shot-to-shot phase. Moreover, an echo-train shifting method is developed with ACE-EPTI to enable imaging at the shortest possible TE for higher SNR. This work has preliminarily validated: i) the high imaging fidelity of ACE-EPTI in a retrospective in-vivo experiment, ii) the higher SNR-efficiency of ACE-EPTI than single-shot EPI (ss-EPI) in phantom and in-vivo experiments, and iii) ACE-EPTI’s ability to provide high-quality diffusion images and diffusion-weighted quantitative maps.
Methods
ACE-EPTI is designed based on the time-resolved imaging concept of EPTI, where multi-contrast images at each echo can be recovered without distortion and blurring by combining spatiotemporal sampling and k-t reconstruction9,12. To improve SNR-efficiency, a continuous “echo-train shifted” readout is utilized (Fig. 1A), where the echo-train is set to begin at spin-echo to minimize TE. This sequence aims at acquiring a series of images across the readout window, starting with T2-weighted SE image followed by images with progressively more T2*-weighting. The optimized 3-shot spatiotemporal encoding (Fig. 1B) is designed with (i) complementary ky sampling along t to control aliasing, (ii) oversampling at central k-space and early TE to improve SNR, (iii) small blips to reduce echo spacing (ESP), (iv) low-frequency signals in each shot for self-navigated physiological phase correction.The low-rank subspace reconstruction13,12 with phase correction is used to recover the image series with improved conditioning. The phase change of each shot is estimated using the low-frequency signals and incorporated into the reconstruction. Moreover, the reconstruction employs complex temporal bases considering both B0 and T2*, which enables the estimation of high-resolution image phases. After reconstruction, distortion-free blurring-free images at different TEs are obtained, DW-T2* and tissue phase can also be calculated.
All the experiments were performed on a 3T Prisma scanner with a 32-channel receiver coil.
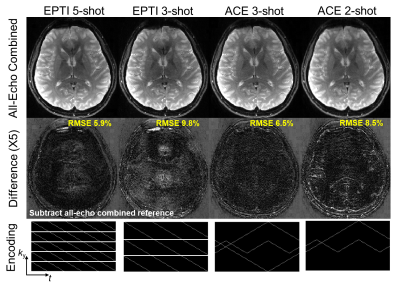
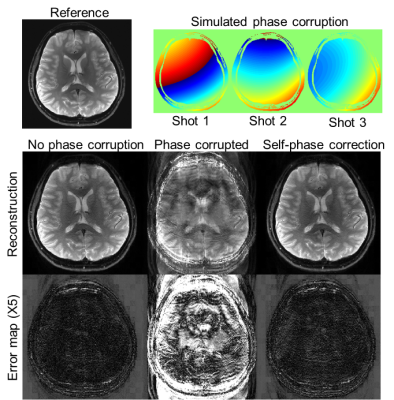
Retrospective undersampling experiment: Fully-sampled non-DW k-t data were acquired at 0.86×0.86 mm2 to evaluate the proposed encoding for ACE-EPTI. The data were undersampled to mimic different acquisitions: 5-shot EPTI, 3-shot EPTI, 3-shot and 2-shot ACE-EPTI. The proposed self-navigated phase correction was also evaluated by adding spatially-varying random phase to different shots.
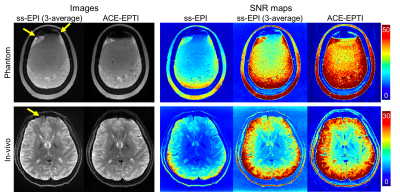
SNR comparison: Phantom and in-vivo data were acquired to evaluate the SNR of ACE-EPTI compared with ss-EPI at 0.86×0.86×3 mm3. 40 dynamics were acquired for 3-shot ACE-EPTI and 120 dynamics for ss-EPI at b-value=0 s/mm2. ACE-EPTI was acquired with a TEmin=31ms for SE and a TErange of 31-92ms, while the ss-EPI was acquired with a TE of 57ms using partial-Fourier 6/8 and Rinplane of 4.
Diffusion acquisition: 18-direction diffusion data were acquired using 3-shot ACE-EPTI with the following parameters: resolution=0.86×0.86×3 mm3, NSA=3, TR=3s, b-value=1000 s/mm2, TErange=38-99ms (ss-EPI with PF=6/8 and Rinplane=4 will have a TE of 63ms), acquisition time = 8 minutes.
Results
Figure 2 shows the reconstructed images and error maps from the retrospective undersampling experiment, where the echo-combined images from EPTI and ACE-EPTI acquisitions were compared with the all-echo combined fully-sampled reference. Overall, 3-shot ACE-EPTI achieves higher accuracy than the other 2- and 3-shot strategies, providing high-quality images close to the 5-shot EPTI. Figure 3 shows the results from the shot-to-shot phase correction test, which demonstrates the proposed self-navigated correction can effectively eliminate phase variation artifacts.Figure 4 shows the reconstructed images and SNR maps from ss-EPI and 3-shot ACE-EPTI in phantom and in-vivo experiments. ACE-EPTI not only avoids the distortion artifacts of ss-EPI (yellow arrows), but also achieves 30-40% higher SNR when compared against a time-matched 3-average ss-EPI, demonstrating the higher SNR-efficiency achieved by ACE-EPTI through echo-train shifting.
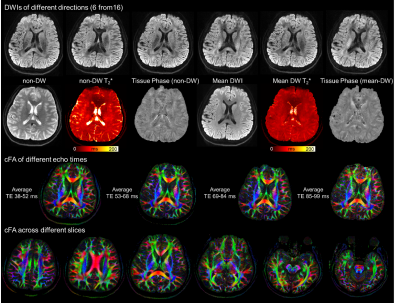
Figure 5 shows the results of in-vivo diffusion imaging using ACE-EPTI. The reconstructed DWIs and FAs show high image quality and resolution. Moreover, T2* and tissue phase maps were also obtained with and without diffusion weighting, as well as FA maps at different echo times. The DW-T2* generally appears lower than the non-DW-T2*, which potentially could be due to the reduction in the extracellular water that has longer T2* than axonal and myelin water.
Conclusion
ACE-EPTI was demonstrated to be a powerful and versatile technique for high-resolution diffusion MRI and diffusion-relaxometry, which provides high SNR-efficiency, high image quality without distortion/blurring, self-navigated phase correction, capability for time-resolved multi-contrast imaging, and fast acquisition with the 3-shot scheme.Acknowledgements
This work was supported by the NIH NIBIB (R01-EB020613, R01-EB019437, R01-MH116173, R01EB016695, P41EB030006, and U01-EB025162) and the instrumentation Grants (S10-RR023401, S10-RR023043, and S10-RR019307).References
1. Chen NK, Guidon A, Chang HC, Song AW. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). NeuroImage 2013;72:41-47.
2. Dong Z, Wang F, Ma X, Zhang Z, Dai E, Yuan C, Guo H. Interleaved EPI diffusion imaging using SPIRiT‐based reconstruction with virtual coil compression. Magn Reson Med. 2018;79(3):1525-1531.
3. Jeong HK, Gore JC, Anderson AW. High-resolution human diffusion tensor imaging using 2-D navigated multishot SENSE EPI at 7 T. Magn Reson Med. 2013;69(3):793-802.
4. Zahneisen B, Aksoy M, Maclaren J, Wuerslin C, Bammer R. Extended hybrid-space SENSE for EPI: Off-resonance and eddy current corrected joint interleaved blip-up/down reconstruction. NeuroImage 2017;153:97-108.
5. Cao X, Liao C, Zhang Z, Iyer SS, Wang K, He H, Liu H, Setsompop K, Zhong J, Bilgic B. T2-BUDA-gSlider: rapid high isotropic resolution T2 mapping with blip-up/down acquisition, generalized SLIce Dithered Enhanced Resolution and subspace reconstruction. arXiv preprint. arXiv:1909.12999 (2019).
6. In MH, Posnansky O, Speck O. High-resolution distortion-free diffusion imaging using hybrid spin-warp and echo-planar PSF-encoding approach. NeuroImage 2017;148:20-30.
7. Zaitsev M, Hennig J, Speck O. Point spread function mapping with parallel imaging techniques and high acceleration factors: fast, robust, and flexible method for echo-planar imaging distortion correction. Magn Reson Med. 2004;52(5):1156-1166.
8. Dong Z, Wang F, Reese TG, Manhard MK, Bilgic B, Wald LL, Guo H, Setsompop K. Tilted-CAIPI for highly accelerated distortion-free EPI with point spread function (PSF) encoding. Magn Reson Med. 2019;81(1):377-392.
9. Wang F, Dong Z, Reese TG, Bilgic B, Katherine Manhard M, Chen J, Polimeni JR, Wald LL, Setsompop K. Echo planar time-resolved imaging (EPTI). Magn Reson Med. 2019;81(6):3599-3615.
10. Fair MJ, Wang F, Dong Z, Reese TG, Setsompop K. Propeller echo-planar time-resolved imaging with dynamic encoding (PEPTIDE). Magn Reson Med. 2020;83(6):2124-2137.
11. Fair MJ, Liao C, Manhard MK, Setsompop K. Diffusion‐PEPTIDE: Distortion‐ and blurring‐free diffusion imaging with self‐navigated motion‐correction and relaxometry capabilities. Magn Reson Med. 2020.
12. Dong Z, Wang F, Reese TG, Bilgic B, Setsompop K. Echo planar time-resolved imaging with subspace reconstruction and optimized spatiotemporal encoding. Magn Reson Med. 2020.
13. Liang Z-P. Spatiotemporal imaging with partially separable functions. 2007. IEEE. p 988-991.
Figures