1313
Dynamic parallel transmission for diffusion MRI at 7T
Belinda Ding1, Iulius Dragonu2, Patrick Liebig3, Robin M Heidemann3, and Christopher T Rodgers1
1Wolfson Brain Imaging Centre, University of Cambrige, Cambridge, United Kingdom, 2Siemens Healthcare Limited, Firmley, United Kingdom, 3Siemens Healthineers, Erlangen, Germany
1Wolfson Brain Imaging Centre, University of Cambrige, Cambridge, United Kingdom, 2Siemens Healthcare Limited, Firmley, United Kingdom, 3Siemens Healthineers, Erlangen, Germany
Synopsis
In this study, we showed the novel application of dynamic pTx pulses for diffusion MRI on a Siemens 7T Terra scanner, with an 8Tx32Rx Nova head coil. We compared the performance of subject-specific spokes pulses against traditional circularly polarised pulses in a healthy volunteer. We observed that pTx pulses improves the signal across the whole brain, especially in lower brain regions like the cerebellum. This leads to improved definition of diffusion tracts and higher FA values in the regions of interest. In conclusion, this work demonstrated the feasibility and benefits of using pTx pulses for diffusion MRI at 7T.
Introduction
Since its introduction in 1985, diffusion MRI (dMRI) has enabled the visualisation and characterisation of brain white matter, allowing scientists and clinicians to study and diagnose diseases including multiple sclerosis, stroke and dementia1 and to study the brain’s structural connectivity (“wiring”). One of the main challenges of dMRI is its intrinsic low signal-to-noise ratio (SNR), which can be improved by scanning at ultra-high field strength2. However, the increased RF transmit (B1+) non-uniformity at 7T often results in spatially non-uniform signal magnitude across the brain, including signal dropouts in lower brain regions3. This is particularly problematic in dMRI since typical sequences rely on a high flip angle (nominally 180°) refocusing pulse. In this abstract, we tackle the challenge of B1+ non-uniformity using RF parallel transmission (pTx) by demonstrating the use of dynamic pTx spokes pulses in dMRI (for the first time to our knowledge) and comparing performance against dMRI using only pulses played in the Circularly Polarised (CP+) mode that is equivalent to a traditional single-channel transmit system.Methods
Data acquisition: One healthy volunteer (female, age = 28 years old) gave informed consent and was scanned in a 7T Terra scanner (Siemens) with an 8Tx/32Rx head coil (Nova Medical). A T1 -weighted whole brain structural image was obtained with a MP2RAGE sequence at 0.75 mm isotropic resolution. dMRI data were collected with a single‐shot, spin‐echo, echo planar imaging (EPI) sequence modified to enable pTx pulses. Diffusion-weighting gradients were applied in 6 directions with a b value of 1000 s/mm2; TE = 60 ms; FOV = 225 × 225 mm2; voxel size = 1.5 × 1.5 × 1.5 mm3; 80 slices with 20% gap covering the entire brain; bandwidth = 1852 Hz/Px; GRAPPA acceleration factor R=3 and 6/8 partial Fourier. For each diffusion‐weighted acquisition, one image without diffusion weighting (b = 0) was also acquired with matching imaging parameters. The entire diffusion protocol was acquired using CP excitation/refocusing, and then repeated with dynamic pTx spokes for both excitation and refocusing. In CP mode, all 80 slices were acquired in one scan, however, due to the conversative SAR limits imposed in pTx mode, the 80 slices were acquired in 4 groups of 20 slices each. On top of that, to limit central brightening artifact in CP acquisition, the flip angles used in both CP and pTx acquisitions were reduced to 80° and 140° for excitation and refocusing respectively. An additional pair of EPI images were acquired for distortion correction purposes with matching imaging parameters but opposite phase encoding directions.Pulse design: For the pTx acquisition, spokes pulses were designed separately for excitation and refocusing. B0 field-maps and per-channel B1+ field-maps were acquired using the default vendor provided sequences embedded in the pTx framework. The field-maps were exported and used for offline MATLAB pulse design. For the two superior imaging slabs, one set of pulses (excitation and refocusing) were designed per slab. For the two inferior imaging slabs, four sets of pulses (excitation and refocusing) were designed per slab to combat the greater variation in B1+ in the inferior brain. Thus, a total of 10 sets of pulses (10 excitation pulses and 10 refocusing pulses) were designed across the 80 slices. i.e. one pair of pulses for each 20 slices in the superior slabs, and one pair of pulses for each 5 slices in the inferior half of the imaging volume. Each pulse consists of 3 spokes with positions determined using the inverse Fourier transform method. Channel weightings were optimised by a magnitude least square algorithm4. The pulses were then converted to variable-rate selective excitation (VERSE) pulses5 to reduce both SAR and pulse duration (Figure 1).
Data analysis: The DTI data were analysed in FSL. They were first distortion-corrected using ‘topup’6 before measures derived from the diffusion MR data, including frational anisotropy (FA) and mean diffusitivity (MD), were calculated using ‘dtifit’. For ROI analysis, the JHU white-matter tractography atlas7 was used and registration between the MNI 152 common space and the DTI images was performed via the T1 structural image using an affine transformation (using ‘flirt’)8.
Results
CP pulses yielded lower signal intensity and signal dropouts in the brain, especially in the cerebellum, inferior temporal lobes and brainstem (Figure 2). Tissue contrasts in these areas are subsequently recovered with pTx pulses. Violin plots also show increased signal intensities across all ROIs (Figure 3).The signal dropouts in the CP acquisition translate into noisy depiction of the FA distribution in these regions (Figure 4). By contrast, the diffusion tracts in the pTx acquisition are much more defined, especially in the brainstem. The average FA values are also higher when using spokes pulses compared to CP pulses across all ROIs (Figure 5).
Discussion & Conclusions
The preliminary results from this experiment show the potential value of dynamic pTx pulses for dMRI. Compared to the standard CP pulses, pTx pulses increases signal across the whole brain potentially leading to a better definition of fibre orientations, especially in problematic areas such as the temporal lobes or cerebellum. This could allow improved visualisation of white matter architecture in those basal areas with ultra-high field strength.Acknowledgements
BD is supported by Gates Cambridge Trust. CTR is funded by a Sir Henry Dale Fellowship from the Wellcome Trust and the Royal Society [098436/Z/12/B]. This study was funded by the NIHR Cambridge Biomedical Research Centre and MRC Clinical Research Infrastructure Award for 7T and has also received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 801075.References
- Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: A review. J Mol Neurosci. 2008;34(1):51-61. doi:10.1007/s12031-007-0029-0
- Wu W, Poser BA, Douaud G, et al. High-resolution diffusion MRI at 7T using a three-dimensional multi-slab acquisition. Neuroimage. 2016;143:1-14. doi:10.1016/j.neuroimage.2016.08.054
- Wu X, Auerbach EJ, Vu AT, et al. High-resolution whole-brain diffusion MRI at 7T using radiofrequency parallel transmission. Magn Reson Med. 2018;80(5):1857-1870. doi:10.1002/mrm.27189
- Setsompop K, Wald LL, Alagappan V, Gagoski BA, Adalsteinsson E. Magnitude least squares optimization for parallel radio frequency excitation design demonstrated at 7 Tesla with eight channels. Magn Reson Med. 2008;59(4):908-915. doi:10.1002/mrm.21513
- Conolly S, Nishimura D, Macovski A, Glover G. Variable-rate selective excitation. J Magn Reson. 1988;78(3):440-458. doi:10.1016/0022-2364(88)90131-X
- Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. Neuroimage. 2003;20(2):870-888. doi:10.1016/S1053-8119(03)00336-7
- Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36(3):630-644. doi:10.1016/j.neuroimage.2007.02.049
- Jenkinson M, Bannister P, Brady M, Smith S. Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. Neuroimage. 2002;17(2):825-841. doi:10.1006/nimg.2002.1132
Figures
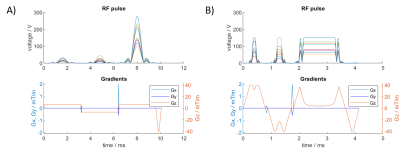
Figure 1: An example of 3 spokes refocusing pulse before (A) and
after (B) conversion into a VERSE pulse.
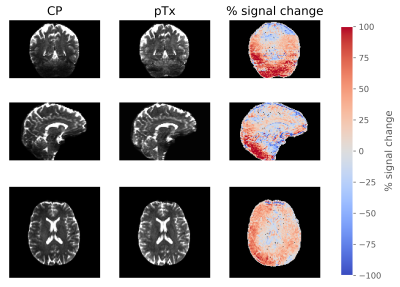
Figure 2: Direct comparison of the b=0 s/mm2
images between CP and pTx acquisition in three orientations. The right most
column shows the percentage signal change between the pTx and CP acquisition.
The signal change was calculated as 200% × [signal(pTx) – signal(CP)] /
[signal(pTx) + signal(CP)] .
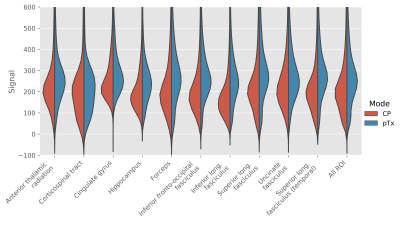
Figure 3: Violin plot showing distribution of signal
intensity in b=0 s/mm2 acquisition across the various ROIs in the
JHU atlas.
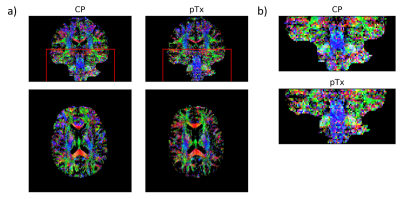
Figure 4: (a) Colour FA maps acquired with CP pulses
(left) and pTx spokes pulses (right). The maps show FA (in the range of [0.25
1]) with colours representing the orientation of the first eigenvector (red:
left-right; green: anterior-posterior; blue: inferior-superior). (b) Zoomed
region of interest as denoted by the red box in (a). Diffusion fibres are more
clearly seen in the pTx acquisition especially in the brainstem.
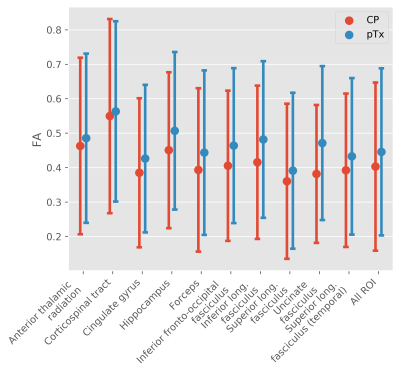
Figure 5: Mean and standard deviation in FA in the various
ROIs for both acquisitions.