1308
Interhemispheric Functional Connectivity and 18F-fluatmetamol PET differentiate AD, amyloid and non-amyloid Mild cognitive decline1The University of Hong Kong, Hong Kong, Hong Kong, 2Queen Mary Hospital, Hong Kong, Hong Kong, 3Imperial College London, London, United Kingdom
Synopsis
Interhemispheric functional connectivity and 18F-flutametamol PET
Introduction
Previous studies [1], [2] have demonstrated that accumulations of amyloid fibrils (AF) altered the cognitive performance in patients suffered from Alzheimer’s disease (AD) and Mild cognitive impairment (MCI).Method
Seventy participants (16 AD, 11 MCI with amyloid positive (amyMCI),18 MCI with amyloid negative (NamyMCI) and 25 healthy control (HC)) were recruited from the university hospital memory clinic. A multidisciplinary panel formed by a neuroradiologist and two geriatricians classified each of them into AD, amyMCI, NamyMCI based on clinical history, neuropsychological score (HK-MoCA), structural MRI and MR perfusion [3]. MRI images were acquired using a Phillips Achieva 3T. A T1W MPRAGE (repetition time [TR]=6.8ms, echo time [TE]=3.2ms, inversion time [TI]=844ms, flip angle = 8o, 256 slices; field of view = 256mm, voxel size = 1 x 1 x 1.2mm) and one 8-min resting state scan (multi-echo echo planar imaging (EPI) sequence; 180 time points; TR=2000ms Flip angle = 90o, 36 slices, voxel size = 1.6 x 1.6 x 4mm). Each participants were asked to remain quiet and relax during the scan, with their eyes closed but not to fall asleep. The fMRI images were pre-processed by SPM12 with Matlab 2018a. It included elimination of the first 10 time points, slice timing correction, normalization and head motion correction. For VMHC, we adopted the method of calculation used by Kelly et al 2011 [4] and Zuo et al 2010 [5]. VMHC map was obtained. Two sample T-test was performed to compare each group with HC. Two-tailed Gaussian Random Field theory with voxel level of p<0.01 and cluster level of p<0.05 was used. PET images were acquired using General Electric PET/CT scanner, using 18-F Flutametamol as tracer. Standardized uptake value (SUV) were retrieved from the PET images. Voxel by voxel correlation analysis between IFC and SUV were calculated using DPARSF ver 4.0, with age, gender and total intracranial volume as covariates.Result
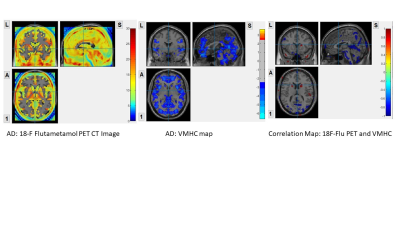
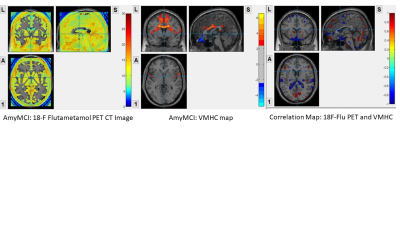
In AD group, high amyloid load was found at precuneus, cuneus, calcarine, hippocampus, parahippocampus, middle cingulate, lingual gyrus, inferior temporal gyrus, inferior occipital gyrus, posterior central gyrus and superior parietal gyrus. These regions also showed attenuated IFC in VMHC map. The correlation map showed negative correlation in these regions, suggested positive amyloid load with lowered IFC in these regions.In AmyMCI group, the amyloid load was lower when compared to AD group. Relatively high amyloid load at middle frontal orbital gyrus, olfactory, precuneus, cuneus, lingual gyrus, calcarine; low amyloid load at middle cingulate and supplementary motor area. Attenuated IFC at middle frontal orbital gyrus; increase IFC were found at middle cingulate, supplementary motor area. The correlation map showed negative correlation at olfactory, middle frontal orbital, middle cingulate and supplementary motor area suggested positive amyloid load with lowered IFC. Positive correlation map showed at most mild amyloid load regions including calcarine, lingual gyrus, anterior cingulate, superior medial frontal gyrus, cuneus and precuneus, suggested that mild amyloid load may induce IFC increase.
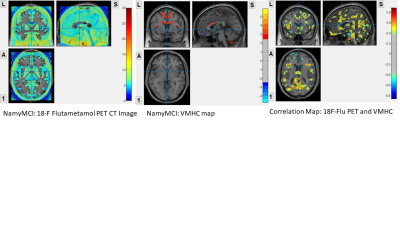
Low amyloid load in NamyMCI group when compared to AD and AmyMCI group. Low amyloid load at middle cingulate and supplementary motor area. Increase IFC were found at these two regions. The correlation map showed positive correlation, at posterior cingulate, precuneus, cuneus, middle cingulate, supplementary motor area, superior medial frontal gyrus, olfactory and rectus, suggested that very low level of amyloid load may induce IFC increase at these regions.
Conclusion
The voxel by voxel correlation analysis showed that high amyloid load will attenuated IFC, while low amyloid load will promote IFC. The finding suggested that IFC can be a non-invasive diagnostic tool for patient with early dementia, who have low amyloid load and showed high IFC in the VMHC map.Acknowledgements
This study was supported by the State Key Laboratory of Brain and Cognitive Science, the University of Hong Kong.References
[1] W. E. Klunk, “Binding of the Positron Emission Tomography Tracer Pittsburgh Compound-B Reflects the Amount of Amyloid- in Alzheimer’s Disease Brain But Not in Transgenic Mouse Brain,” J. Neurosci., vol. 25, no. 46, pp. 10598–10606, Nov. 2005, doi: 10.1523/JNEUROSCI.2990-05.2005.
[2] D. R. Thal et al., “[ 18 F]flutemetamol amyloid positron emission tomography in preclinical and symptomatic Alzheimer’s disease: Specific detection of advanced phases of amyloid-β pathology,” Alzheimers Dement., vol. 11, no. 8, pp. 975–985, Aug. 2015, doi: 10.1016/j.jalz.2015.05.018.
[3] B. Dubois et al., “Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria,” Lancet Neurol., vol. 6, no. 8, pp. 734–746, Aug. 2007, doi: 10.1016/S1474-4422(07)70178-3.
[4] C. Kelly et al., “Reduced interhemispheric resting state functional connectivity in cocaine addiction,” Biol. Psychiatry, vol. 69, no. 7, pp. 684–692, Apr. 2011, doi: 10.1016/j.biopsych.2010.11.022.
[5] X.-N. Zuo et al., “Growing Together and Growing Apart: Regional and Sex Differences in the Lifespan Developmental Trajectories of Functional Homotopy,” J. Neurosci., vol. 30, no. 45, pp. 15034–15043, Nov. 2010, doi: 10.1523/JNEUROSCI.2612-10.2010.