1304
QSM detects early alterations of brain venous blood oxygenation in fetuses with complex congenital heart diseases1Radiology, Shandong Medical Imaging Research Institute, Cheeloo College of Medicine, Shandong University, Jinan, China, 2Ultrasound, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, China, 3Radiology Research, Children’s Hospital of Philadelphia, Philadelphia, PA, United States, 4MR Collaboration, Healthcare Siemens Ltd., Beijing, China, 5MR Application, Siemens Healthineers Ltd., Jinan, China, 6Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
We investigated the early changes of brain oxygenic metabolism in fetuses with complex congenital heart disease (CHD) and in normal fetuses across gestational ages. Using quantitative susceptibility mapping (QSM), we measured the venous blood oxygen saturation (SvO2) in utero of 22 fetuses with complex CHD and 88 healthy pregnancy controls. The SvO2 in normal fetuses was found to have no age-related change. SvO2 values were significantly higher in the CHD fetuses (80.8%±4.6%) than in the gestational age-matched normal fetuses (75.7%±8.0%) (p=0.038); which is evidence of altered human fetal brain oxygenic metabolism during the early stages of brain development.
Introduction
Congenital heart disease (CHD), a common congenital deformity, has a prevalence of approximately 0.6%-0.8% in live neonates. A widely discussed mechanism implies that complex CHD in fetuses can change the hemodynamics and blood oxygen level of the brain, which may result in impaired neurodevelopment. However, little is known about the timing of altered brain oxygenic metabolism of fetuses with complex CHD, which ultimately may affect brain growth. This study investigated the early changes of venous blood oxygen saturation (SvO2) in fetuses with complex CHD and in normal pregnancies across gestational ages (GAs) by using quantitative susceptibility mapping (QSM) in utero. We hypothesize that complex CHD impacts the oxygenic metabolism of fetuses at an early stage, resulting in increased SvO2 values compared with values in healthy control fetuses.Methods
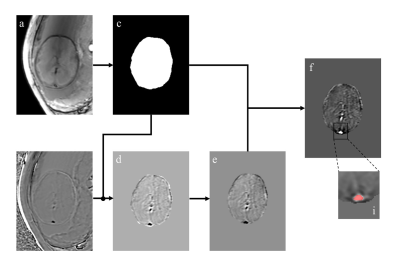
In this prospective study, in vivo 3D susceptibility-weighted imaging (SWI) was performed in 88 normal fetuses (GA: 30.5±3.8 weeks, range 21.6–37.9 weeks) and 22 CHD fetuses (GA: 26.3±2.2 weeks, range 23.0–29.6 weeks). The SWI examinations were performed on a 3 T MR scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) with an 18-channel body coil. The acquisition location was axial planar of the fetal brain. The 3D SWI sequence was conducted in one breathhold, and both magnitude images and phase images were acquired. The detailed parameters for the 3D-SWI sequence were repetition time = 16 ms; echo time = 11.1 ms; flip angle = 10°; acquisition matrix = 448×360; slice thickness = 3.5 mm; number of slices=16-18; band width=220 Hz/Px; voxel size reconstructed to 0.8 mm × 0.8 mm × 3.5 mm; and acquisition time = 17s. QSM images were reconstructed from the SWI phase images to quantify the corresponding SvO2 of the superior sagittal sinus through a modified QSM processing pipeline (http://martinos.org/~berkin/software.html), including (1) Laplacian unwrapping; (2) manual delineation of the fetal brain for mask creation; (3) Laplacian boundary value determination to remove background field, followed by 3D polynomial fit; and (4) construction of thresholded k-space division algorithm to generate QSM (Figure 1). The association between SvO2 and GA was assessed with a linear regression model for the fetuses with CHD and normal fetuses. The comparison of the SvO2 between fetuses with CHD and normal fetuses was conducted in GA-matched fetuses (GA:20-30 weeks), using analysis of covariance.Results
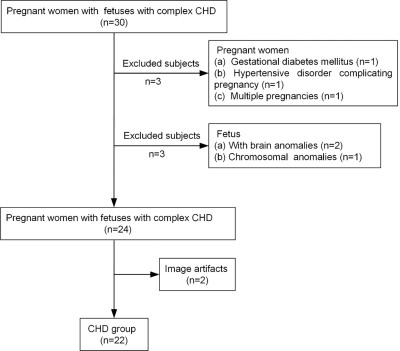
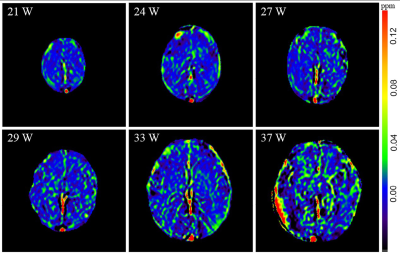
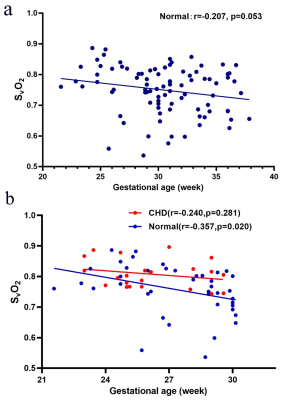
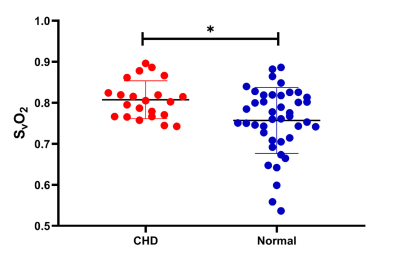
There was no significant difference in GA between the normal group and the CHD group (p=0.054). A flowchart of selection of the CHD participants is presented in Figure 2. The CHD structural lesions consisted of complete transposition of great arteries (6/22, 27.3%); hypoplastic left heart syndrome (4/22, 18.2%); critical aortic stenosis (4/22, 18.2%); tetralogy of Fallot (2/22, 9.09%); severe pulmonary stenosis or atresia (1/22, 4.55%); single cardiac ventricle (1/22, 4.55%); total anomalous pulmonary venous drainage (1/22, 4.55%); right ventricular dysplasia (1/22, 4.55%); and hypoplastic aortic arch (2/22, 9.09%). Figure 3 presents the QSM maps of the normal group by GA. Figure 4a presents the SvO2 alterations across GAs from 21 to 38 weeks in the normal group; the SvO2 had a downward but not statistically significant trend across GA (p=0.053). Forty-two GA-matched normal fetuses (GA: 27.4±2.4 weeks, range: 20-30 weeks) were selected for comparison of the SvO2 of CHD fetuses. Figure 4b shows the SvO2 alterations across GAs from 20 to 30 weeks in the CHD group and the GA-matched normal group and their corresponding fitting curve. In the normal matched group, SvO2 decreased significantly as GA advanced (p=0.022), whereas SvO2 did not change significantly with GA in CHD fetuses (p=0.281). Figure 5 presents the SvO2 value in the CHD group and GA-matched normal group. The average Δχv of the superior sagittal sinus in normal fetuses (0.230±0.083 ppm) was higher than in CHD fetuses (0.183±0.045 ppm) across all GAs (p=0.015). The analysis of covariance revealed that the corresponding putative SvO2 was significantly different between the CHD (80.8%±4.6%) group and the GA-matched normal group (75.7%±8.0%) after the effects of GA were excluded (p=0.038).Discussion and Conclusion
In this in vivo study, we quantified (with QSM) the SvO2 values of fetuses with complex CHD between 20 and 30 GA weeks and those of normal pregnancy during the second and third trimester. The SvO2 values of CHD fetuses were higher than in normal fetuses even after the effects of GA were excluded. The presence of SvO2 abnormalities between 20 and 30 weeks in the fetuses that have CHD is presumptive evidence of altered fetal brain oxygenic metabolism during the early stages of human brain development. The possible significance of previously unrecognized abnormally high SvO2 in fetuses with CHD on brain development deserves investigation.Acknowledgements
This work is made possible by the following funding support: National Natural Science Foundation of China (81671668).References
1.Matthiesen NB, Henriksen TB, Agergaard P, et al. Congenital Heart Defects and Indices of Placental and Fetal Growth in a Nationwide Study of 924 422 Liveborn Infants. Circulation. 2016;134(20):1546–1556. doi: 10.1161/CIRCULATIONAHA.116.021793.
2.Khalil A, Bennet S, Thilaganathan B, Paladini D, Griffiths P, Carvalho JS. Prevalence of Prenatal Brain Abnormalities in Fetuses with Congenital Heart Disease: Systematic Review. Ultrasound Obstet Gynecol. 2016;48(3):296-307. doi: 10.1002/uog.15932.
3.Sun L, Macgowan CK, Sled JG, et al. Reduced Fetal Cerebral Oxygen Consumption Is Associated With Smaller Brain Size in Fetuses With Congenital Heart Disease. Circulation. 2015;131(15):1313–1323. doi: 10.1161/CIRCULATIONAHA.114.013051.
4.Peyvandi S, Latal B, Miller SP, McQuillen PS. The neonatal brain in critical congenital heart disease: Insights and future directions. NeuroImage. 2019;185:776–782. doi: 10.1016/j.neuroimage.2018.05.045.
5.Schellen C, Ernst S, Gruber GM, et al. Fetal MRI detects early alterations of brain development in Tetralogy of Fallot. American Journal of Obstetrics and Gynecology. 2015;213(3):392.e1-392.e7. doi: 10.1016/j.ajog.2015.05.046.
6.Yadav BK, Buch S, Krishnamurthy U, et al. Quantitative susceptibility mapping in the human fetus to measure blood oxygenation in the superior sagittal sinus. Eur Radiol. 2019;29(4):2017–2026. doi: 10.1007/s00330-018-5735-1.
7.Yadav BK, Hernandez-Andrade E, Krishnamurthy U, et al. Dual-Imaging Modality Approach to Evaluate Cerebral Hemodynamics in Growth-Restricted Fetuses: Oxygenation and Perfusion. Fetal Diagn Ther. 2020;47(2):145–155. doi: 10.1159/000500954.
8.Yadav BK, Krishnamurthy U, Buch S, et al. Imaging putative foetal cerebral blood oxygenation using susceptibility weighted imaging (SWI). Eur Radiol. 2018;28(5):1884–1890. doi: 10.1007/s00330-017-5160-x.
Figures