1297
Regional Brain Growth in Fetuses with Congenital Diaphragmatic Hernia1Radiology, Boston Children's Hospital, Boston, MA, United States, 2Radiology, Harvard Medical School, Boston, MA, United States, 3Universidad del Rosario, Bogota, Colombia
Synopsis
Brain growth trajectories of fetal subjects with congenital diaphragmatic hernia (CDH) were compared to typically developing fetuses. T2-weighted SSFSE acquisitions were reconstructed to a super-resolution volume and atlas-based segmentations of brain structures were propagated. Gestational age, sex, segment volumes, and morphological hernia metrics (for CDH subjects) were analyzed in 71 fetuses with CDH and in 50 controls. We found lower brain volumes relative to normal fetuses in the developing white matter, hippocampus, diencephalon, and deep gray structures. Metrics associated with greater hernia severity were associated with lower volumes. Furhermore, our analysis shows that right-sided hernias were associated with lower volumes.
Introduction
Congenital Diaphragmatic Hernia (CDH) is a developmental defect of the diaphragm that permits abdominal organs to herniate into the thoracic cavity. A growing body of evidence reports abnormalities in fetal brain MRI of subjects with CDH, including larger cerebrospinal fluid (CSF) spaces and volume loss1. Prior studies of fetuses with CDH performed qualitative analyses or limited measurements to explore these emerging differences. This study used computational neuroimaging tools to characterize the regional specificity and time of onset of abnormalities in the brain of fetuses with CDH and investigated the relationship with morphologic parameters of the hernia.Materials and Methods
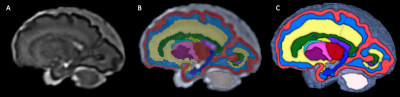
This study was HIPAA compliant and IRB approved. We retrospectively analyzed images of fetuses with a diagnosis of CDH that underwent MRI at our hospital for medical reasons. Criteria for inclusion were a) singleton pregnancy between 20 and 40 weeks of gestational age (GA) and b) CDH. Criteria for exclusion were a) syndromic etiology, b) coexistence of other major fetal abnormalities (e.g. congenital heart disease). For control subjects, we analyzed images of typically developing fetuses recruited for a separate study on normal fetal brain growth.MRI acquisition and processing: All examinations and studies for both groups were performed on 3-Tesla Siemens MRI scanners and included multi-planar T2-weighted single-shot fast spin-echo (SSFSE) sequences, effective echo time 100 - 120 ms; repetition time of 1400-2000 ms; and 2-4 mm slice thickness. The T2 SSFSE images for all cases were processed using a previously validated algorithm that performed super-resolution volume reconstruction with inter-slice motion correction, and intensity normalization2. The reconstructed images were registered to a fetal brain MRI atlas in a common coordinate space3. Subsequently, multi-atlas label fusion was performed to generate labels for the individual subjects as described by Gholipour et al. 20173; these were then revised by a pediatric neuroradiologist with experience in fetal imaging. Labels for 26 regions of interest were propagated (Figure 1, see results section).
Hernia Parameters: All CDH subjects underwent same day detailed ultrasonography (US) survey. A pediatric radiologist evaluated the fetal body MRI and US images and determined: a) hernia side, b) herniated stomach, liver, or bowel, c) lung-to-head circumference ratio (LHR), d) observed/expected LHR (OELHR), e) percent predicted lung volume (PPLV), and f) amniotic fluid index.
We evaluated GA as a predictor of volume with a linear regression analysis, accounting for sex as a covariate. Estimated GA coefficients were compared between groups by seemingly unrelated estimation and a Wald chi-square test. For areas with significant differences, we performed a separate linear regression analysis with backward stepwise selection to investigate the association between morphological parameters of the hernia and brain volumetry.
Results
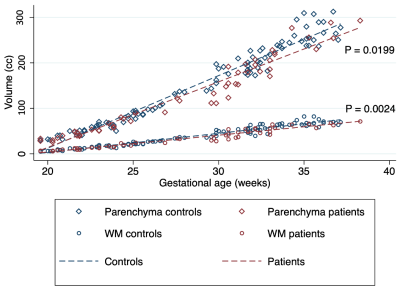
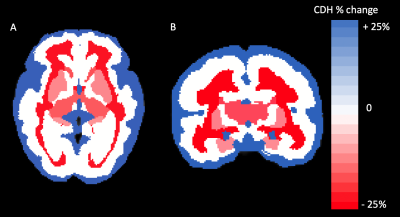
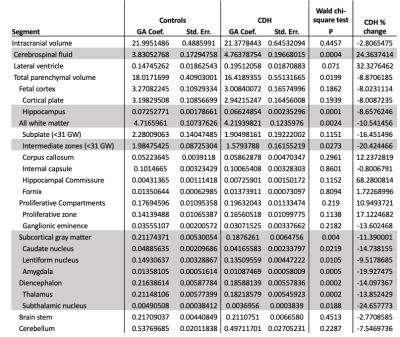
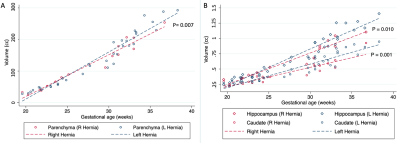
We analyzed MRIs of 71 fetuses with CDH (45 male; GA 29.1 weeks SD 5.2) and 50 controls ( 20 male; GA 28.0 weeks SD 5.3). 35 fetuses had left-sided hernias. CDH fetuses had significantly higher CSF volume (P = 0.0004), with a mean increase of 24% relative to controls. Conversely, CDH was associated with lower total parenchymal volume (P = 0.0199), developing white matter volume (P = 0.0024), subcortical gray matter volume (P = 0.004), and diencephalic volume (P = 0.0002) (Figure 2). Percent differences in these ranged between -8.9% and -14.1% (Figure 3). For details see Figure 4. The hippocampus was significantly smaller in CHD fetuses (P = 0.0001). In subjects < 31 weeks, we saw significantly lower volume in the intermediate zone (P = 0.0273). All constituents of the deep gray matter and the diencephalon were significantly smaller in CDH (all P < 0.0219).Fetuses with right sided hernias had significantly lower total brain volume (β -30.89, P = 0.007), hippocampal volume (β -0.14, P = 0.010), and caudate volume (β -0.16, P = 0.001) (Figure 5). Higher total parenchymal volume, developing white matter volume, hippocampal volume, and caudate volume were associated with higher OELHR and lower LHR (all P < 0.050).
Discussion
Fetuses with CDH have global and regional differences in brain volume relative to normal fetuses which are mediated by lower volumes in the developing white matter (i.e., intermediate zone in those <31 weeks), hippocampus, diencephalon, and deep gray structures. The precise etiology is unknwon but a decrease in substrate delivery (lower cardiac output) secondary to compression of the heart by the hernia has been postulated4. The increase in CSF is consistent with the results of Radhakrishnan et al.1, and probably reflects the parenchymal volume loss. The pattern of abnormality is specific to CDH, as it differs from that observed in congenital heart disease where the subplate and the proliferative zones are prominently affected5. Metrics of hernia severity (OELHR and LHR) predicted lower parenchymal volume which suggests that prenatal neurodevelopmental abnormalities could be contributing to the association of these metrics with worse outcomes. Finally, fetuses with right-sided CDH had lower parenchymal volume, suggesting differences in the pathophysiology of brain injury which could be due to a different effect on the heart or to differences in the genotypes predisposing to hernias in this location.Conclusion
Fetuses with CDH show region-specific patterns of volume loss which correlate with metrics of hernia severity and are influenced by the side of the hernia.Acknowledgements
Research reported in this study was supported in part by the National Institute of Biomedical Imaging and Bioengineering and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (NIH) under Award Numbers R01EB018988, R01NS106030, and R01EB013248; by a Technological Innovations in Neuroscience Award from the McKnight Foundation; Schlaeger Fellowship for Neuroscience Research; and by the Fetal Health Foundation (FHF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, FHF, or the McKnight Foundation.References
1. Radhakrishnan, R. et al. Fetal brain morphometry on prenatal magnetic resonance imaging in congenital diaphragmatic hernia. Pediatr Radiol 49, 217–223 (2019).
2. Kuklisova-Murgasova, M., Quaghebeur, G., Rutherford, M. A., Hajnal, J. V. & Schnabel, J. A. Reconstruction of fetal brain MRI with intensity matching and complete outlier removal. Medical Image Analysis 16, 1550–1564 (2012).
3. Gholipour, A. et al. A normative spatiotemporal MRI atlas of the fetal brain for automatic segmentation and analysis of early brain growth. Sci Rep 7, 476 (2017).
4. Van Mieghem, T. et al. Left ventricular cardiac function in fetuses with congenital diaphragmatic hernia and the effect of fetal endoscopic tracheal occlusion. Ultrasound Obstet Gynecol 34, 424–429 (2009).
5. Rollins, C. K. et al. Regional Brain Growth Trajectories in Fetuses with Congenital Heart Disease. Ann Neurol (2020) doi:10.1002/ana.25940.
Figures