1294
Diagnostic accuracy of ASL in comparison with DSC perfusion in the surveillance of different types of brain tumors
Anna Lavrova1, Wouter Teunissen2, Esther Warnert2, Martin van den Bent3, Vladimir Cheremisin1, and Marion Smits2
1Radiology, Saint Petersburg University, Saint Petersburg, Russian Federation, 2Radiology, Erasmus MC, Rotterdam, Netherlands, 3Neurology, Erasmus MC, Rotterdam, Netherlands
1Radiology, Saint Petersburg University, Saint Petersburg, Russian Federation, 2Radiology, Erasmus MC, Rotterdam, Netherlands, 3Neurology, Erasmus MC, Rotterdam, Netherlands
Synopsis
Dynamic susceptibility contrast-enhanced (DSC) perfusion is an established standard in the assessment of brain tumor perfusion. This study aims to assess the feasibility of using non-contrast arterial spin-labeling (ASL) instead of DSC.
Introduction
Dynamic susceptibility contrast-enhanced (DSC) perfusion for a long time remains an established standard in the assessment of brain tumors. Modern MRI techniques, such as arterial spin-labeling (ASL), doesn’t require intravenous contrast administration and thus, could challenge DSC, which could be extremely important for patients, who have an allergy, kidney insufficiency, or require many follow-up scans. This study aims to assess the feasibility of the use of ASL perfusion instead of DSC in the surveillance of different types of primary and metastatic brain tumors.Methods
We included 122 (78 males, mean age 54±13) patients in this retrospective study, who underwent both ASL and DSC perfusion in the same MRI-scanning session at 3T (GE) during 2019. ASL was acquired as a 3D PCASL sequence with spiral readout and background suppression using 111° FA, TE = 10.6 ms, TR = 4635 ms, 4 mm slices, and single PLD (1.5 s), voxel sizes were 1.9 x 1.9 x 3.5 mm3. DSC was performed approximately 5 minutes after a full-dose preload bolus, with another bolus of gadolinium-based contrast agent using 90° FA, TE = 45 ms, TR = 2 s, voxel sizes were 2 x 2 x 5 mm3. ASL-derived Cerebral Blood Flow (CBF) maps were created with Ready View (AW Server, GE); DSC-derived relative Cerebral Blood Volume (rCBV) maps, uncorrected for leakage, were created with Intellispace Portal (Philips). Lesions were identified as T2 and T2-FLAIR hyperintensity with or without contrast enhancement (CE), as well as areas of highest signal intensity on the perfusion maps, i.e., “hot spots’’. Measurements were done in Radiant DICOM Viewer by placing a region of interest of approximately 70 mm2 on the rCBV map and copying it to the contralateral normal-appearing white matter (NAWM) and to the CBF map. Pearson’s correlation coefficient testing was done on the CBF and rCBV ratios of tumor versus NAWM.Results
We identified 183 lesions, 120 with and 63 without CE. Correlation coefficients were as follows: glioma with (80 lesions, r=0.648, p<0.001), and without CE (56 lesions, r=0.551, p<0.001), lymphoma with (10 lesions, r=-0.233, p=0.464) and without CE (7 lesions, r=0.02, p=0.952), metastases with CE (30 lesions, r=0.33, p=0.081).Conclusion
We found a strong correlation in glioma, while there was no significant correlation in lymphoma and metastases, which could be related to positive therapeutic response and will be further investigated in the follow-up analysis. Our findings so far suggest that ASL can be used instead of DSC to measure perfusion in glioma at 3T.Limitations
Cases with severe artifacts, such as movements (one case) and signal loss at the site of the tumor (two cases), were excluded from the analysis. Also, we acknowledge that the statistical analysis could be affected due to the low NAWM signal to noise ratio on CBF maps.Acknowledgements
I would like to express my very great appreciation to all the co-authors for their tremendous contribution to this study and all the time support.References
No references found.Figures
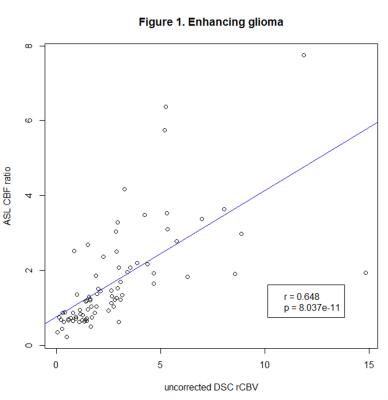
Figure 1. Pearson’s correlation coefficients of ASL CBF ratio and uncorrected DSC rCBV in enhancing glioma.
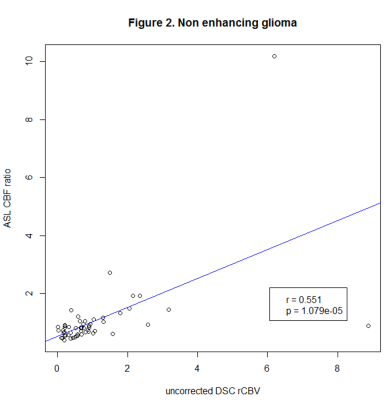
Figure 2. Pearson’s correlation coefficients of ASL CBF ratio and uncorrected DSC rCBV in non-enhancing glioma.
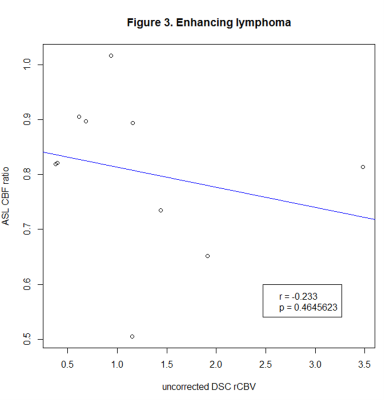
Figure 3. Pearson’s correlation coefficients of ASL CBF ratio and uncorrected DSC rCBV in enhancing lymphoma.
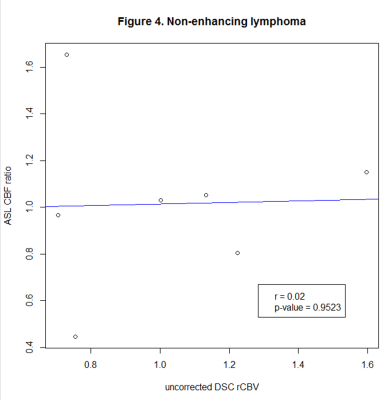
Figure 3. Pearson’s correlation coefficients of ASL CBF ratio and uncorrected DSC rCBV in non-enhancing lymphoma.
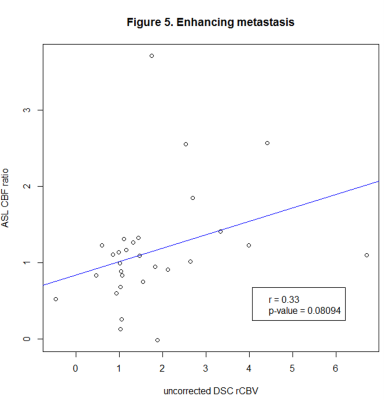
Figure 5. Pearson’s correlation coefficients of ASL CBF ratio and uncorrected DSC rCBV in enhancing metastases.