1273
Multi-contrast Brain Image Registration for Low-contrast Paediatric Magnetic Resonance Imaging1Melbourne Neuropsychiatry Centre, The University of Melbourne, Parkville, Australia, 2Department of Electrical and Electronic Engineering, The University of Melbourne, Parkville, Australia, 3Psychiatry Monash Health, Faculty of Medicine, Nursing and Health Sciences School of Clinical Sciences, Monash University, Melbourne, Australia
Synopsis
A multi-contrast brain imaging technique has been presented, that leverages information from T1- and T2-weighted images to register iso-intense paediatric MRI data. The proposed method is applied to T1-weighted images of 6-months old infants, and the performance is evaluated in comparison to commonly employed single-contrast registration techniques.
Introduction
Despite the potential of paediatric MRI to investigate healthy and perturbed brain development, paediatric image processing pipelines are scarce. The majority of well-established pipelines for processing adult brains perform sub-optimally on paediatric images, due to changes in contrast, size and shape and degree of myelination1. Processing MRI data from infants aged 6 months or less is particularly challenging due to iso-intense images with extremely low contrast between white and gray matter structures2,3.Brain image registration is a necessary process to perform spatial normalization across different subjects or time. During a typical scanning session, multiple MRI sequences with complimentary contrast information are acquired, and longitudinal studies typically scan participants at multiple time-points. Here, we propose a multi-contrast image registration method (MCR) that combines information from multi-sequence MRI to improve registration accuracy in matching neuro-anatomical landmarks between images across subjects and time-points. We demonstrate the utility of the proposed method by registering T1w images from 6-month old infants to a publicly available paediatric brain template, and quantitively compare MCR to commonly employed single-contrast registration techniques.
Theory and methods
Multi-contrast optimizationConsider a multi-contrast set of images, $$$I=\{I_1,...,I_N\}$$$, acquired in a single scanning session, and a set of reference images, $$$R=\{R_1,...,R_N\}$$$, where $$$N$$$ is the number of contrasts in the data. An image, $$$I_i$$$,$$$I\in\{1,...,N\}$$$, can be registered to a reference image, $$$R_i$$$, using a two-component symmetric normalization (SyN) process: a shape transformation model and a weighted similarity space4: $$\text{arg}\min_v\left(\int_{0}^{1}{\parallel}v(x,t)\parallel_Ldt+\sum_{i=1}^Nw_i\psi_i\right) ,$$ the first component is the shape transformation model, $$$v(x,t)$$$ is a time-varying velocity field and $$$L$$$ is an appropriate norm. The second component is a weighted sum of similarity metrics, $$$\psi_i$$$, across $$$N$$$ contrasts. The similarity term can include multiple metrics, such as cross-correlation, mutual information and intensity differences.
Participants
This study includes a subset of infants (n=6, age 5.83$$$\pm$$$0.75 months) from an NIH-funded Autism centers of Excellence (AcE) network Infant Brain Imaging Study (IBIS)5. Data collection sites had study protocols approval from their Institutional Review Boards, and all enrolled subjects had informed consent provided by parent/guardian.
MRI acquisition
MRI scans were performed on 3T Siemens Tim Trio scanners with 12-channel head coils while infants were naturally sleeping. The multi-sequence imaging protocol included: T1-weighted imaging (T1w), 3D T1 MPRAGE sequence, TR=2,400ms, TE=3.16ms, matrix=160x224x256, voxel size=1x1x1mm3. T2-weighted imaging (T2w), 3D T2 FSE sequence, TR=3,200ms, TE=499ms, matrix=160x256x256, voxel size=1x1x1 mm3. Details of full imaging protocol are described elsewhere5.
Multi-contrast registration
The multi-contrast image registration pipeline (MCR) for T1w images was implemented using Advanced Normalization Tools (ANTs) version 2.3.16. T1w and T2w templates from a publicly available paediatric atlas (4.5-8.5 years) were used as reference images7. Prior to registration, all T1w image intensities were winsorized at the 99.5th percentile, and histogram matching was performed between the input and reference images.
Multistage linear transformation of the individual T1w images into the reference space included: 1) an intensity-based initial geometric translation, 2) a rigid transformation and 3) an affine transformation. Rigid/affine transformation parameters: gradient step=0.1, linear interpolation, mutual information as similarity metric, bin-size=32, regular sampling strategy (100% sampling), convergence threshold=1e-6, convergence window size=10, 4 levels with number of iterations=1000x500x250x100, shrink factors=8x4x2x1, gaussian-smoothing with $$$\sigma$$$=3x2x1x1xvoxel-size (reference space).
The multi-contrast SyN optimization consisted of two cross-correlation based similarity terms. The first term maximized the similarity between $$$(I_{T_1w}, R_{T_1w})$$$, with parameters: metric-weight=2, radius=8. The second term maximized the similarity between $$$(I_{T_2w}, T_{T_2w})$$$, with metric-weight=1, radius=8. The transform parameters were: gradient-step=0.5, updateFieldVarianceInVoxelSpace=5, 3 levels with number of iterations=1200x1200x100, convergence threshold=1e-6, convergence window-size=5, shrink-factors=8x6x2, gaussian-smoothing with $$$\sigma$$$=8x4x2xvoxel-size (reference space).
Skull-stripping
To perform skull-stripping, individual images were registered to T1w template image using MCR, and the brain mask in the reference space was inverse transformed to each individual image. The individual brain masks were visually inspected for correctness.
Validation
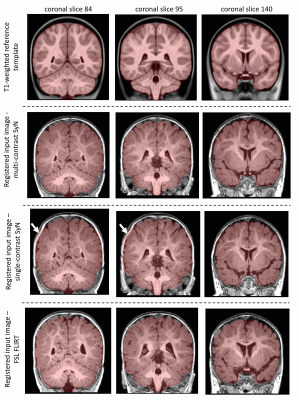
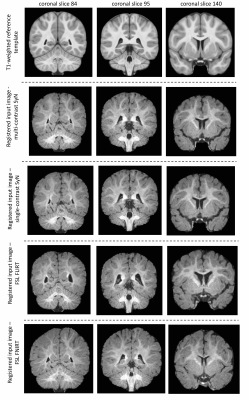
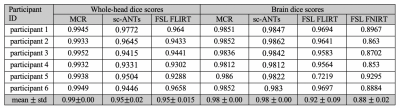
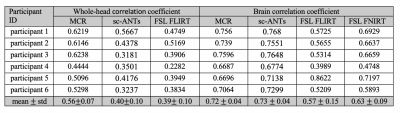
For comparison, single-contrast registration was performed between T1w individual and template images using single-contrast ANTs (sc-ANTs) and FLIRT8 with and without skull-stripping. Additionally, FNIRT9 was implemented on skull-stripped data, with parameter adjustment for infant brain size. Quantitative evaluation in the reference space (T1w template) included: 1) dice scores between template and individual brain masks to assess brain surface matching, 2) correlation coefficient between whole-brain template and individual image intensities.
Results and discussion
Performance of multi-contrast registrationMCR on whole-head images showed improved performance in matching brain boundaries to the template image (Figure 1). MCR outperformed sc-ANTs, FLIRT and FNIRT in matching anatomical boundaries in skull-stripped data (Figure 2). All methods showed limited performance in white-matter structures, potentially due to lack of myelination and the wide age-range in the template.
Quantitative evaluation in reference space
The dice-index of MCR-derived brain masks was consistently higher on whole-head (0.99$$$\pm$$$0.00) and skull-stripped (0.98$$$\pm$$$0.00) data, compared to whole-head sc-ANTs (0.95$$$\pm$$$0.01), FLIRT (0.95$$$\pm$$$0.015) and skull-stripped sc-ANTs (0.98$$$\pm$$$0.00), FLIRT (0.92$$$\pm$$$0.09) and FNIRT (0.88$$$\pm$$$0.02) (Table 1). The correlation-coefficient between T1w template and MCR-registered intensities described stronger correlations in whole-head (0.56$$$\pm$$$0.07) and skull-stripped (0.71$$$\pm$$$0.04) images, compared to whole-head sc-ANTs (0.4$$$\pm$$$0.09), FLIRT (0.39$$$\pm$$$0.1) and skull-stripped sc-ANTs (0.73$$$\pm$$$0.03), FLIRT (0.57$$$\pm$$$0.15) and FNIRT (0.63$$$\pm$$$0.09) (Table 2).
Conclusion
A multi-contrast image registration technique was proposed to achieve improved spatial normalization in low-contrast paediatric MRI images. Future work aims to include diffusion MRI-based contrasts to improve performance in white-matter structures, and to develop multi-contrast segmentation techniques.Acknowledgements
No acknowledgement found.References
[1] Phan, T. V., Smeets, D., Talcott, J. B., & Vandermosten, M. (2018). Processing of structural neuroimaging data in young children: Bridging the gap between current practice and state-of-the-art methods. Developmental Cognitive Neuroscience, 33(December 2016), 206–223.
[2] Zhang, W., Li, R., Deng, H., Wang, L., Lin, W., Ji, S., & Shen, D. (2015). Deep convolutional neural networks for multi-modality isointense infant brain image segmentation. NeuroImage, 108, 214–224.
[3] Dubois, J., Alison, M., Counsell, S. J., Lucie, H. P., Hüppi, P. S., & Benders, M. J. N. L. (2020). MRI of the Neonatal Brain: A Review of Methodological Challenges and Neuroscientific Advances. Journal of Magnetic Resonance Imaging.
[4] Avants, B. B., Epstein, C. L., Grossman, M., & Gee, J. C. (2008). Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis, 12(1), 26–41.
[5] Hazlett, H. C., Ph, D., Gu, H., Ph, D., Mckinstry, R. C., Ph, D., Shaw, D. W. W., Botteron, K. N., Dager, S. R., Styner, M., Ph, D., Vachet, C., Gerig, G., Ph, D., Paterson, S. J., Ph, D., Schultz, R. T., Ph, D., Estes, A. M., … Piven, J. (2012). Brain Voulme Findings in 6-Month-Old Infants at High Familial Risk for Autism. Am J Psychiatry 2012.
[6] Avants, B. B., Tustison, N. J., Song, G., Cook, P. A., Klein, A., & Gee, J. C. (2011). A Reproducible Evaluation of ANTs Similarity Metric Performance in Brain Image Registration. NeuroImage, 54(4), 2033–2044.
[7] Fonov, V.S., et al., Unbiased average age-appropriate atlases for pediatric studies. NeuroImage, 2011(54): p. 313-327
[8] Greve, D.N. and Fischl, B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage, 48(1):63-72, 2009.
[9] M. Jenkinson, C.F. Beckmann, T.E. Behrens, M.W. Woolrich, S.M. Smith. FSL. NeuroImage, 62:782-90, 2012
Figures