1269
The Use of the 3He/129Xe MRI Lung Morphometry for a Longitudinal Observation of the Emphysema Progression in AATD Patients1Physics and Astronomy, Western University, London, ON, Canada, 2Lawson Health Research Centre, London, ON, Canada, 3Department of Medical Biophysics, Western University, London, ON, Canada, 4Department of Medicine, Respirology, Western University, London, ON, Canada, 5Robarts Research Institute, London, ON, Canada, 6School of Biomedical Engineering, Western University, London, ON, Canada
Synopsis
In this study, we demonstrated a possible solution to underestimation of the global mean that can mask the severity of emphysema progression. Four patients with Alpha-1 Anti-Trypsin Deficiency disorder (AATD) were measured in 2014 using hyperpolarized He to measure lung function, and again in 2018 to measure lung function. While it is evident looking at morphometry images that there is a significant reduction in pixels and therefore emphysema progression, it is not at first evident in morphometry estimates. By normalizing these morphometry estimates of ADC/Lm, we can better characterize emphysema progression in individuals with AATD
Purpose
Hyperpolarized gas pulmonary MRI1,2 provides physiologically relevant biomarkers (apparent-diffusion-coefficient (ADC) and mean linear intercept estimate (Lm) of obstructive lung disease including emphysema, bronchopulmonary dysplasia, congenital lobar emphysema and alpha-1 antitrypsin deficiency (AATD).3-5 However, the longitudinal observation of the emphysema progression using hyperpolarized gas MRI-based ADC/Lm can be problematic, as the disease progression can lead to increasing unventilated lung areas, which likely excludes the largest ADC/Lm estimates. This can result in underestimation of the global mean ADC/Lm values, masking the emphysema severity. Clearly, the static-ventilation measurements providing the gas distribution should still show an increase in the ventilation defects reflecting emphysema progression, but these measurements do not provide quantitative information about the lung parenchyma microstructure. One solution to this problem is to combine static-ventilation and morphometry measurements; however, the current approach requires two separate scans using two hyperpolarized gas doses (causing slice mismatch) and different voxel sizes, so combining such measurements is not an easy task. We have previously developed a lung morphometry method allowing us to acquire static-ventilation images and ADC/morphometry maps during a 16sec single breath-hold.6,7 We hypothesize that this morphometry method should help to overcome the above-mentioned shortcomings and provide an accurate assessment of the emphysema progression. For this work, we used the static-ventilation and ADC morphometry data acquired using the traditional approach (3He data, 2014) and recently proposed approach (129Xe data, 2014). A small group of AATD subjects were involved for four years of hyperpolarized gas pulmonary studies focused on emphysema progression.Methods
Methods: Four AATD patients provided written informed consent to an ethics-board approved study protocol and underwent spirometry, plethysmography, and 3He/129Xe MRI morphometry twice within a 4-year interval. 3He MRI was performed at 3.0T (MR750, GEHC, WI) using whole-body gradients (5G/cm maximum) and a commercial, rigid linear human RF coil (Rapid Biomedical, Germany). For 3He MRI scans a single breath-hold, five interleaved acquisitions (TE=4.1msec, TR=6.0msec, reconstructed matrix size=128x128, number of slices=15; slice thickness=15mm, and FOV=40x40cm2) with and without diffusion sensitization were acquired for a given line of k-space to ensure that RF depolarization (4o constant-flip-angle was used). The diffusion-sensitization gradient pulse ramp up/down time=500μs, constant time=460μs and diffusion time (Δ)=1.46ms resulted in images acquired at five different b-values: 0, 1.6, 3.2, 4.8 and 6.4s/cm2. 3He static ventilation imaging was performed as previously described.3 129Xe MRI was performed at 3.0T using whole-body clinical gradients and a commercial, xenon quadrature flex human RF coil8 (MR Solutions, USA). For xenon measurements, the diffusion-sensitization gradient pulse ramp up/down time=500μs, constant-time=2ms, ΔXe=5.2ms, providing five b-values 0, 12.0, 20.0, 30.0, and 45.5s/cm2. To accelerate 129Xe morphometry acquisition, an under-sampled (AF=7) multi-slice interleaved (six interleaves) centric 2D FGRE diffusion-weighted sequence was acquired, for seven 30mm coronal-slices (TE=10msec, TR=13msec, reconstructed matrix size=128x128, and FOV=40x40cm2, 14sec single breath-hold).9 An extra interleave with no diffusion-weighting (b=0) and significantly reduced TE (2ms) was utilized to generate a short-TE static-ventilation-image.9 A 7.4o constant-flip-angle (120 [20 per b-value] RF pulses-per-slice) was used for the AF=7.9 The 3He/129Xe morphometry maps and ADC (b=0/b=16s/cm2 and b=0/b=12s/cm2) were generated for all cases as previously described.3,6,7,10-14 3He/129Xe static-ventilation imaging was performed as previously described.3 and the ventilation defect percentage (VDP) was calculated.15Results
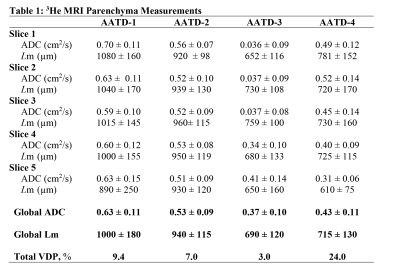
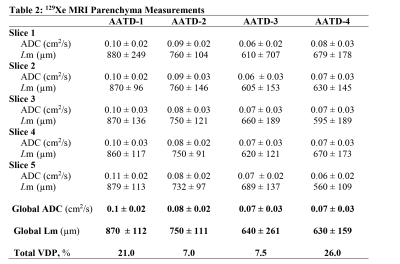
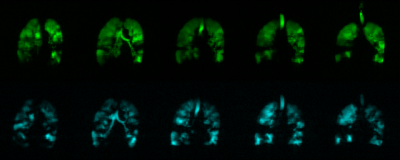
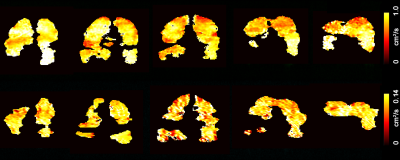
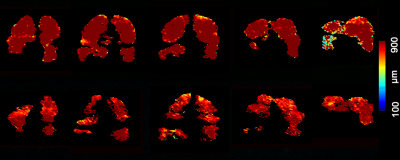
Figure 3 shows five representative static-ventilation slices obtained for the AATD-1 subject with 3He (top-panel, 2014) and 129Xe (bottom-panel, 2018). The VDP estimated for all subjects are summarized in Figures 1 and 2. Figures 4 and 5 show five representative ADCHe/ADCXe and LmHe/LmXe maps for the AATD-1 subject while Figures 1 and 2 show the five-slice mean estimates obtained with 3He (top-panel, 2014) and 129Xe (bottom-panel, 2018) for all AATD subjects.Discussion and Conclusion
In this study, we showed that the emphysema progression over the 4-year term can be quantified by using pulmonary static ventilation and diffusion-weighted images of hyperpolarized gas, but not without some modifications to the original estimates. The estimates generated from the static-ventilation imaging shows lung health worsening over the study term and the emphysema biomarkers generated from the diffusion-weighted imaging suggest a slow disease progression. The increased number of the ventilation defects can be clearly seen in Figure 1 as well as the significantly reduced number of pixels on ADC/Lm maps (Figures 2 and 3) following the increase of the ventilation defects. There were, however, some notable decreases in ADC/Lm measurements, which indicates an overall decrease in emphysema progression. One possible solution to this problem is to normalize the ADC/Lm by the VDP and thus take into account the decrease in ventilated lung volume. The feasibility of such an approach has been recently demonstrated using the 3He static-ventilation and diffusion-weighted data.6 Our 3He datasets were acquired using two separate breath-holds (two helium doses) and different voxel-sizes between static-ventilation and diffusion-weighted data so, the slice resolution difference and slice mismatch make this task very difficult. Our 129Xe datasets were acquired using a single breath-hold accelerated imaging approach allowing static-ventilation and ADC/Lm mapping simultaneously6,7 so, there are no slice resolution difference and slice mismatch. For future work, we plan to normalize the 129Xe ADC/Lm estimates by 129Xe VDP for an accurate assessment of the emphysema progression.Acknowledgements
A. Ouriadov was funded in part by a fellowship from the Alpha-1 Foundation (USA).References
1 Mugler, J. P., 3rd & Altes, T. A. Hyperpolarized 129Xe MRI of the human lung. J Magn Reson Imaging 37, 313-331, doi:10.1002/jmri.23844 (2013).
2 Driehuys, B. et al. Chronic obstructive pulmonary disease: safety and tolerability of hyperpolarized 129Xe MR imaging in healthy volunteers and patients. Radiology 262, 279-289, doi:10.1148/radiol.11102172 (2012).
3 Kirby, M. et al. Hyperpolarized 3He and 129Xe MR imaging in healthy volunteers and patients with chronic obstructive pulmonary disease. Radiology 265, 600-610, doi:10.1148/radiol.12120485 (2012).
4 Kaushik, S. S. et al. Diffusion-weighted hyperpolarized 129Xe MRI in healthy volunteers and subjects with chronic obstructive pulmonary disease. Magn Reson Med 65, 1154-1165, doi:10.1002/mrm.22697 (2011).
5 Kirby, M. et al. Hyperpolarized 3He and 129Xe magnetic resonance imaging apparent diffusion coefficients: physiological relevance in older never- and ex-smokers. Physiol Rep 2, doi:10.14814/phy2.12068 (2014).
6 Westcott, A., Guo, F., Parraga, G. & Ouriadov, A. Rapid single-breath hyperpolarized noble gas MRI-based biomarkers of airspace enlargement. J Magn Reson Imaging 49, 1713-1722, doi:10.1002/jmri.26574 (2019).
7 Ouriadov, A., Guo, F., McCormack, D. G. & Parraga, G. Accelerated 129Xe MRI morphometry of terminal airspace enlargement: Feasibility in volunteers and those with alpha-1 antitrypsin deficiency. Magnetic Resonance in Medicine 84, 416-426, doi:10.1002/mrm.28091 (2020).
8 Kaushik, S. S. et al. Single-breath clinical imaging of hyperpolarized (129)Xe in the airspaces, barrier, and red blood cells using an interleaved 3D radial 1-point Dixon acquisition. Magn Reson Med 75, 1434-1443, doi:10.1002/mrm.25675 (2016).
9 Ouriadov, A., Guo, F., McCormack, D. G. & Parraga, G. Accelerated 129Xe MRI Morphometry of Terminal Airspace Enlargement: Feasibility in Volunteers and Those with Alpha-1 Antitrypsin Deficiency. Mag Res in Med, doi:10.1002/MRM.28091 (2019).
10 Chan, H. F., Stewart, N. J., Parra-Robles, J., Collier, G. J. & Wild, J. M. Whole lung morphometry with 3D multiple b-value hyperpolarized gas MRI and compressed sensing. Magn Reson Med 77, 1916-1925, doi:10.1002/mrm.26279 (2017).
11 Yablonskiy, D. A. et al. Quantification of lung microstructure with hyperpolarized 3He diffusion MRI. J Appl Physiol (1985) 107, 1258-1265, doi:10.1152/japplphysiol.00386.2009 (2009).
12 Sukstanskii, A. L. & Yablonskiy, D. A. Lung morphometry with hyperpolarized 129Xe: theoretical background. Magn Reson Med 67, 856-866, doi:10.1002/mrm.23056 (2012).
13 Ouriadov, A., Lessard, E., Sheikh, K., Parraga, G. & Canadian Respiratory Research, N. Pulmonary MRI morphometry modeling of airspace enlargement in chronic obstructive pulmonary disease and alpha-1 antitrypsin deficiency. Magn Reson Med 79, 439-448, doi:10.1002/mrm.26642 (2018).
14 Ouriadov, A. et al. Pulmonary hyperpolarized 129Xe morphometry for mapping xenon gas concentrations and alveolar oxygen partial pressure: Proof-of-concept demonstration in healthy and COPD subjects. Magn Reson Med 74, 1726-1732, doi:10.1002/mrm.25550 (2015).
15 Kirby, M. et al. Hyperpolarized 3He magnetic resonance functional imaging semiautomated segmentation. Acad Radiol 19, 141-152, doi:10.1016/j.acra.2011.10.007 (2012).
Figures