1267
Towards correlating tissue status with dynamic PRF-T1 measurements using a single reference dual flip angle technique
Henrik Odéen1, Sara L Johnson1, Allison H Payne1, and Dennis L Parker1
1Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States
1Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States
Synopsis
Simultaneous PRF/T1 measurements using a single reference variable flip angle method is used to investigate the utility of T1 as a complimentary measure to thermometry for treatment outcome in MR guided thermal interventions, such as focused ultrasound. Both temperature and thermal dose have been shown to be inaccurate indicators of treatment outcome, creating a potential need for alternative quantitative metrics. This can be of particular value in adipose tissues where PRF has very low sensitivity. It is shown that the slope of the T1 vs. temperature curve correlates with amount of delivered thermal dose.
Introduction
There has been increasing development of MRI-guided minimally invasive thermal therapies that utilize real-time MR proton resonance frequency (PRF) thermometry techniques. It has been shown that there are several other quantitative parameters that can inform treatment monitoring and assessment biomarkers, including T1, T2, T2*, ADC and signal intensity (1–3). Quantitative parameter maps can be acquired with high accuracy and precision with dedicated pulse sequences, such as inversion recovery and multi-echo spin echo methods for T1- and T2-maps, respectively, but these methods are typically slow and provides inadequate spatial coverage, limiting their utility to MRI-guided thermal therapies. Obtaining dynamic quantitative T1 and T2 measurements in conjunction with PRF MR thermometry measurements has been demonstrated in vivo (4,5) for focused ultrasound (FUS) applications. However, these methods were based on the dual-flip angle method resulting in long acquisition times. A single reference flip angle technique demonstrated that dynamic hybrid PRF and quantitative T1 measurements could be achieved with a clinically relevant temporal resolution, however, studies relating the T1 measurements to temperature or thermal dose are missing (6). This study demonstrates a single reference approach for simultaneous dynamic PRF-T1 acquisition in ex vivo muscle, and investigates temperature rise and T1 as a function of thermal dose, as a possible complimentary approach for treatment end-point evaluation.Methods
Experimental setup: Dynamic simultaneous PRF/T1 measurements were obtained during an MR guided FUS ablation study in ex vivo chicken breasts. Studies were conducted on a 3T MRI (PrismaFit, Siemens Healthcare, Erlangen, Germany) with a preclinical MRgFUS system (Image Guided Therapy, Pessac, France) using a 256-element transducer (f = 1 MHz, acoustic intensity FWHM = 1.8x1.8x8 mm in water). The ex vivo samples (N=4) were placed in a custom holder (Figure 1) coupled to a degassed water column above the transducer. An in-house built single loop receive coil was placed around the tissue holder. In addition, a ring of room-temperature gelatin phantom surrounded the chicken breast to provide a non-heated standard for referenceless PRF thermometry reconstruction (7,8). In Experiment 1 three samples were used and 4 sonications were consecutively applied at 5-mm spacing in each sample with increasing acoustic power levels (10 to 71 eW) with a 19.6 second heat time followed by a 39.2 second cooling interval. In experiment 2 a total of 14 consecutive sonications were performed in one sample at three power levels (29, 52, and 66 eW). All sonications were monitored with a segmented GRE EPI pulse sequence (FOV=128x64x28 mm, 1x2x2 mm resolution interpolated to 1 mm isotropic spacing, TR/TE=28/12 ms, 14 slices, readout bandwidth = 952 Hz/pixel, ETL = 7, FARef/Dyn=5°/25°, 3.92 sec acquisition time). Prior to each sonication, 5 reference images were acquired.Data analysis: PRF temperature maps were reconstructed by baseline subtraction followed by referenceless reconstruction using the gelatin phantom ring surrounding the muscle as reference, Figure 1 (8). Dynamic T1 measurements were reconstructed using a previously described single-reference dual flip angle approach (6). A fiberoptic temperature probe was placed in the muscle to measure the initial temperature, and thermal dose was calculated (9) from PRF measurements. T1 was plotted as a function of temperature and the slope of the change was analyzed for the heating and cooling curves separately. Mean and standard error of a 3x3 voxel ROI around the hottest voxel was calculated for temperature rise and T1.Results
Figure 2 shows the T1 vs. temperature results for increasing power sonications of experiment 1. In row 1, where no thermal dose was accumulated, the T1 to temperature dependence was consistent during both the heating (red) and cooling (blue) periods for each sonication. As thermal dose accumulated, a substantial jump in the dynamic T1 was observed immediately after the sonication was terminated and the T1/temperature curve during cooling demonstrated a lower slope relative to the heating phase. This effect is also seen in experiment 2, where repeated sonications were applied at the same position in the chicken breast. The temperature and T1 response as a function of time are seen in Figure 3 and the relationship between T1 and temperature is detailed for each sonication in Figure 4. For Experiment 1 Figure 5a shows quantitatively that the change in slope of T1 vs temperature is substantially larger at higher thermal dose values. Similar to experiment 1, the T1/temperature slope response curves between the heating and cooling portions of the sonication curve for experiment 2 become increasingly different as thermal dose increases, Figure 5b.Discussion
Results indicate that single reference dynamic T1 measurements may be a complementary metric to PRF temperature measurements. Temperature and thermal dose have been shown to be somewhat inaccurate indicators of true treatment outcome, creating a potential need for additional quantitative metrics. While particularly critical in fat tissues, where lack of hydrogen bonding makes PRF thermometry inaccurate, this type of dynamic measurement could also benefit the monitoring of all tissue types.Conclusions
Simultaneous PRF-T1 measurements using a single reference dual flip angle method has been demonstrated to achieve volumetric measurements in a clinically relevant time. Temperature-dependency of T1 was shown to correlate with the delivered thermal dose in ex vivo tissue.Acknowledgements
This work was supported by NIH grants R37CA224141, R01EB028316, R03EB023712 and S10OD018482.References
1. Hectors SJCG, Jacobs I, Moonen CTW, Strijkers GJ, Nicolay K. MRI methods for the evaluation of high intensity focused ultrasound tumor treatment: Current status and future needs. Magn. Reson. Med. [Internet] 2016;75:302–317. doi: 10.1002/mrm.25758.2. Hazle JD, Stafford RJ PR. Magnetic resonance imaging-guided focused ultrasound thermal therapy in experimental animal models: correlation of ablation volumes with pathology in rabbit muscle and VX2 tumors. J Magn Reson Imaging. 2002;15:185–94.3. Sequeiros RB, Kariniemi J, Ojala R, Chengli L, Haapea M, Sequeiros AB TO. Liver tumor laser ablation – increase in the subacute ablation lesion volume detected with post procedural MRI. Acta Radiol. 2010;51:505–11.4. Todd N, Diakite M, Payne A, Parker DL. Hybrid proton resonance frequency/T1 technique for simultaneous temperature monitoring in adipose and aqueous tissues. Magn. Reson. Med. 2013;69:62–70.5. Jochen Keupp, Steffen Weiss, Jaakko Tolo, Holger Gruell, Edwin Heijman. Simultaneous T2 mapping in Near-Field Subcutaneous Fat Layer and PRFS Temperature Mapping in the Target Region using Fast Interleaved Sequences to Monitor MR-HIFU Sonication. In: ISMRM. Toronto; 2015. p. 4061.6. Svedin BT, Payne A, Parker DL. Simultaneous proton resonance frequency shift thermometry and T 1 measurements using a single reference variable flip angle T 1 method. Magn. Reson. Med. 2019:1–15. doi: 10.1002/mrm.27643.7. Farrer AI, de Bever J, Coats B, Christenson DA, Payne A. Fabrication and Evaluation of Tissue-Mimicking Phantoms for use with MR-ARFI and MRgFUS. In: International Symposium on Therapeutic Ultrasound. Las Vegas; 2014. p. 232.8. Rieke V, Vigen KK, Sommer G, Daniel BL, Pauly JM, Butts K. Referenceless PRF shift thermometry. Magn. Reson. Med. 2004;51:1223–1231. doi: 10.1002/mrm.20090.9. Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int. J. Radiat. Oncol. Biol. Phys. 1984;10:787–800.Figures
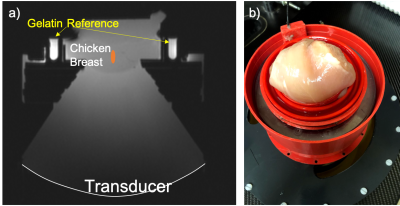
Figure 1. a) Schematic and b) photo
showing the setup with FUS transducer on the bottom and chicken breast at the
top of a water-filled column. A gelatin-filled “rim” was used as reference for
PRF thermometry, and a single loop coil was used for signal reception.
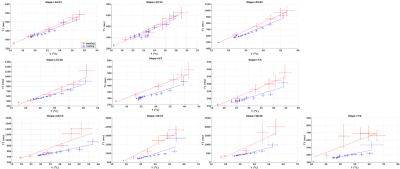
Figure 2. T1 (ms) as
a function of temperature (°C) for points with increasing thermal dose for
experiment 1. Mean +/- standard error over 3x3 voxels is shown for heating
(red) and cooling (blue), and lines indicate linear fits. In the top row 0
CEM43 was delivered, in the middle row between 2 – 15 CEM43 was delivered, and
in the bottom row between 4*107 –
1*1011 CEM43 was delivered. The title for each
sub-plot states the slope of the heating and cooling part, respectively.
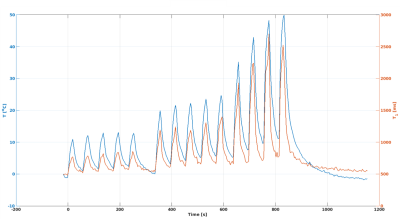
Figure 3. Temperature (°C) and T1 (ms) as
a function of time for experiment 2. The first five sonications used
29 eW, the
next five used 52 eW, and
the last four used 66 eW, all
for 19.6 s with 39.2 s cooling in-between consecutive sonications.
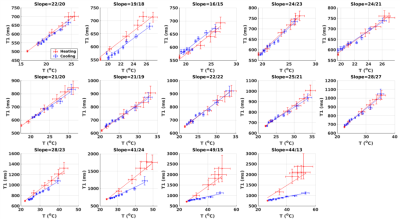
Figure 4. T1 (ms) as
a function of temperature (°C) for the 14 consecutive sonications in
experiment 2. Mean +/- standard error over 3x3 voxels is shown for heating
(red) and cooling (blue), and lines indicate linear fits. The title for each
sub-plot states the slope of the heating and cooling part, respectively. The
last 4 sonications
delivered increasing amounts of dose, from 41 to 1*108 CEM43.
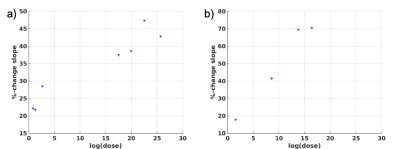
Figure 5. Percent change in slope of T1/T
between the heating and cooling phase of the sonication is plotted as a
function of the logarithm of thermal dose for Experiment 1 (a) and Experiment 2
(b), respectively. The percent change in slope increases with increasing
thermal dose.