1266
Accuracy of MR thermometry during deep radiofrequency hyperthermia treatments in the pelvic region1Department of Radiotherapy, Erasmus MC Cancer Institute, University Medical Center Rotterdam, Rotterdam, Netherlands, 2Department of Radiation Science and Technology, Faculty of Applied Sciences, Delft University of Technology, Delft, Netherlands, 3Center for Care and Cure Technologies Eindhoven (C3Te), Department of Electrical Engineering, Eindhoven University of Technology, Eindhoven, Netherlands
Synopsis
Proton resonant frequency shift is the most frequently used method for MR-thermometry. This method is sensitive to patient motion which poses issues that affect the MR-thermometry accuracy. Few studies have evaluated MR thermometry in the pelvis, but only in volumes far from the regions with air motion, and without an objective patient data exclusion criteria. In this study, we assessed accuracy of MR thermometry for a selected group of patients with cervical carcinoma. We showed that changing gastrointestinal air volume was an important confounder for MR thermometry accuracy and that this can be exploited for selection criteria prior to treatment.
Introduction
The efficacy of hyperthermia depends on the delivery of well controlled and precise heating to the entire tumor volume without overheating the surrounding tissues. Clinical trials have proved that the probability of cure is correlated with the administered thermal dose1,2. The combination of a magnetic resonance (MR)-compatible applicator within an MR-system to monitor the temperature change non-invasively provides an ideal technological platform to achieve dose-optimization in real-time. Proton resonant frequency shift (PRFS)3 is currently the most frequently used method for MR-thermometry but is challenging in the pelvic region. The PRFS method measures temperature changes and it is considerably affected by moving air in the adjacent intestines, causing susceptibility artifacts4. Earlier, a few studies5,6 investigated MR thermometry in the pelvis, but only in volumes far from the regions with air motion, and undefined objective patient data inclusion criteria. In this study, we assessed accuracy of MR thermometry versus intraluminal temperature probes for a homogenous group of patients with cervical carcinoma. We also investigated if intestinal air volume change at the beginning of the treatment could be used for patient selection as predictor for MR thermometry accuracy.Methods
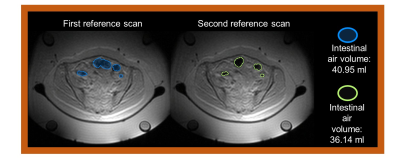
A total of fourteen patients diagnosed with primary or recurrent cervical carcinoma were included in this study. For each patient, on average three hyperthermia treatments were delivered in the BSD‑2000‑3D MR‑compatible system (Pyrexar Medical Corp., Salt Lake City, UT, US)7 integrated in a 1.5 T GE Signa Excite scanner (General Electric Healthcare, Waukesha, USA). We employed the PRFS method, where a dual-echo gradient-echo (GRE) sequence was applied8 (TE=4.8 and 19.1 ms; TR=620 ms; 25 axial slices; slice thickness=1 cm; FOV=50×50 cm2; acquisition matrix=128×128; reconstruction matrix=256×256). Prior to application of RF power, two reference scans were acquired to assess baseline conditions. In addition to the four fat tubes included in the hyperthermia device (Figure 1), body far was used to correct for changes of the static magnetic field B0. A 2D polynomial spatial-temporal correction was applied across the MR temperature maps such that temperature changes were reversed to zero in the selected fat regions. In addition, MR thermometry was filtered such to avoid pollution by data points affected by confounders like motion. The intraluminal thermometry was acquired using high resistive (Bowman) probes. Average temperature was calculated for each time point within the delineated region of interest (ROI) around the probe. MR thermometry accuracy was quantified by the absolute temperature difference between the mean average temperature in the ROI and the corresponding intraluminal temperature measurement. The threshold for acceptable MR thermometry accuracy was < 1°C9. The anatomic images of the reference scans were used to delineate gastrointestinal air (Figure 1 and Figure 2). The gastrointestinal air volume change was quantified by the absolute change between the two reference scans. We selected the treatment sessions based on the absolute gastrointestinal air change below or equal to 5 ml. After this selection, MR thermometry accuracy was evaluated for eight patients (fifteen hyperthermia sessions).Results
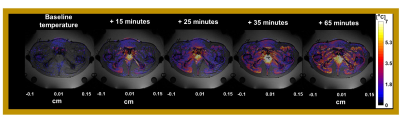
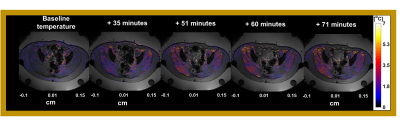
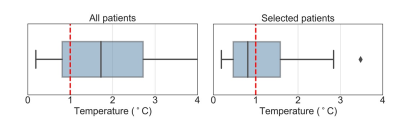
The average of gastrointestinal air volume change in all treatment sessions and in the selected sessions was 20.7± 29.1 ml and 2.3±1.67 ml, respectively. Figure 3 presents the temperature distributions during the hyperthermia treatment of the representative patient. We verified that the gastrointestinal air volume change was 4.8 ml, which represents one of the patients with highest change between the selected patients. Figure 4 presents an example of an unselected patient with a gastrointestinal air volume change of 21.4 ml. As shown, most of the regions where the probes are evaluated were filtered out due to high volumes of air.Figure 5 presents the evaluation of MR thermometry regarding to accuracy in all the patients and in the selected data, where the median accuracy for all patients and selected data was 1.73°C and 0.81°, respectively. Although more than 50% of the selected sessions presented a MR thermometry accuracy within the limits, 46% of the data was outside the acceptable threshold.
Conclusions
Conclusions: Our study showed that change in gastrointestinal air volume could be used as criteria for selection of patients with higher accuracy in MR thermometry. We observed that, in comparison with including all sessions, the selection distinguished a group that has higher MR thermometry accuracy by 53%. This study indicated that changing gastrointestinal air volume was an important confounder for MR thermometry accuracy and that this can be exploited for imaging based selection prior to treatment.Acknowledgements
This research has been made possible by the Dutch Cancer Society and the Netherlands Organization for Scientific Research (NWO) as a part of their joint Partnership Programme: “Technology for Oncology” grant number: 15195 and the Dutch Cancer Society grant KWF-DDHK 2013-6072.References
1. Datta NR, Bodis S. Hyperthermia with radiotherapy reduces tumour alpha/beta: Insights from trials of thermoradiotherapy vs radiotherapy alone. Radiother Oncol. 2019;138:1-8.
2. Datta NR, Stutz E, Gomez S, Bodis S. Efficacy and Safety Evaluation of the Various Therapeutic Options in Locally Advanced Cervix Cancer: A Systematic Review and Network Meta-Analysis of Randomized Clinical Trials. Int J Radiat Oncol Biol Phys. 2019;103(2):411-437.
3. Poorter J De, Wagter C De, Deene Y De, Thomsen C, Ståhlberg F, Achten E. Noninvasive MRI Thermometry with the Proton Resonance Frequency (PRF) Method: In Vivo Results in Human Muscle. Magn Reson Med. 1995;33(1):74-81.
4. Winter L, Oberacker E, Paul K, et al. Magnetic resonance thermometry: Methodology, pitfalls and practical solutions. Int J Hyperth. 2016;32(1):63-75.
5. Gellermann J, Wlodarczyk W, Hildebrandt B, et al. Noninvasive magnetic resonance thermography of recurrent rectal carcinoma in a 1.5 tesla hybrid system. Cancer Res. 2005;65(13):5872-5880.
6. Gellermann J, Hildebrandt B, Issels R, et al. Noninvasive magnetic resonance thermography of soft tissue sarcomas during regional hyperthermia: Correlation with response and direct thermometry. Cancer. 2006;107(6):1373-1382.
7. Gellermann J, Wlodarczyk W, Feussner A, et al. Methods and potentials of magnetic resonance imaging for monitoring radiofrequency hyperthermia in a hybrid system. Int J Hyperth. 2005;21(6):497-513.
8. Curto S, Aklan B, Mulder T, et al. Quantitative , Multi-institutional Evaluation of MR Thermometry Accuracy for Deep-Pelvic MR- Hyperthermia Systems Operating in Multi-vendor MR-systems Using a New Anthropomorphic Phantom. Cancers (Basel). 2019;11:1709. 9. V. V. N. Kothapalli S, Altman MB, Zhu L, et al. Evaluation and selection of anatomic sites for magnetic resonance imaging-guided mild hyperthermia therapy: a healthy volunteer study. Int J Hyperth. 2018;34(8):1381-1389.
Figures