1263
Feasibility of Magnetic Resonance Thermometry at 0.55T1Siemens Medical Solutions USA Inc., Malvern, PA, United States, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Department of Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States
Synopsis
Low field MRI offers advantages such as reduced cost and improved safety of implantable and interventional devices, and reduced RF energy deposition over high field alternatives. However, the accuracy of proton resonance frequency (PRF) thermometry suffers at low field due to reduced signal to noise ratio as well as lower temperature-sensitivity of MR phase. In this study we demonstrate that reduced off-resonance effects, increased T2* and shorter T1 associated with low field can be leveraged to achieve high quality MR thermometry in the brain and prostate at 0.55T.
Introduction
Proton Resonance Frequency (PRF) thermometry is a widely used MR-based technique to monitor changes in tissue temperature in response to thermal therapy1,2. It is used in clinic to guide thermal ablation procedures, for example high intensity focused ultrasound and laser ablation in the brain, and ultrasound ablation in the prostate2. Recent research has demonstrated the feasibility of low field MRI at 0.55T for diagnostic imaging and also suggested its tremendous potential for MR-guided interventions due to significantly reduced device and implant heating, radio-frequency (RF) energy deposition and susceptibility artifacts3. However, PRF thermometry is challenging at 0.55T, due to linear B0 dependence of temperature-induced phase shifts. In this abstract we investigate the feasibility of PRF thermometry in the brain and prostate on a 0.55T whole body prototype system.Methods
PRF thermometry is challenging at 0.55T because both SNR and temperature-induced phase shifts (temperature sensitivity ~0.01 ppm/°C) are inversely proportional to B0. However, low B0 is also associated with longer T2*, reduced susceptibility effects / B0 inhomogeneity, and shorter T1, which can be used to partially compensate for these challenges3. Reduced susceptibility effects enable readouts with higher SNR efficiency, such as segmented EPI, along with reduced receiver bandwidths, to partially compensate for SNR reduction. Longer T2* allows using longer TEs to compensate for reduced temperature sensitivity. Thus, segmented EPI with its ability to enable longer TRs to achieve long TEs without increasing scan time is particularly well suited to benefit from reduced B0 inhomogeneity and long T2* in PRF thermometry at low field. Its combination with 3D acquisition further improves SNR and expands spatial coverage4. The resulting improvement in quality of PRF thermometry is complemented by quicker recovery of longitudinal magnetization due to associated T1 shortening. We have therefore chosen to utilize segmented EPI as the sequence of choice for this study.Data acquisition:
Four healthy volunteers were imaged on a 0.55T MAGNETOM Free.Max prototype (*) (Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, China). All human measurements were performed according to institutional volunteer scanning policies. The following parameters were used to acquire a dynamic series of 80-100 repetitions for assessment of temperature uncertainty:
Brain:
- 2D segmented EPI: TR 75ms, TE 38ms, water excitation, 1.08x2.18x3.5mm3 spatial resolution, 256x128 matrix, echo train length (ETL) 5, readout bandwidth (BW) 130Hz/pixel, temporal resolution 1.95s/slice, flip angle (FA) 40°
- 2D GRE: TR 27ms, TE 12.6ms, 1.08x2.18x3.5mm3 spatial resolution, 256x128 matrix, BW 60Hz/pixel, temporal resolution 3.46s/slice, FA 30°
- 3D segmented EPI: TR 160ms, TE 64ms or 81ms, water excitation, 1.25x2.5x2.5mm3 spatial resolution, 192x96x12 matrix, ETL 33 or 49, BW 130Hz/pixel, temporal resolution 5.8 or 3.8s/volume, FA 40°-55°
- 3D segmented EPI: TR 100ms or 110ms, TE 45ms or 56ms, water excitation, 2x3x3mm3 spatial resolution, 128x128x12 matrix, ETL 23 or 25, BW 398 or 340 Hz/pixel, temporal resolution 7.2 or 6.6s/volume, FA 25°-30°
Processing:
Images or volumes with significant subject motion were removed from each dynamic series using an automatic image similarity-based MATLAB routine. Baseline phase was removed using a Principal Component Analysis based approach5,6, and average phase measured within an SNR-thresholded mask was removed after baseline correction to eliminate global phase drift. Resultant phase difference images were scaled by -1/(γB0 TE×0.01ppm/°C) to estimate temperature difference relative to the first acquisition. Since a temperature change of 0°C is expected in absence of external heating, the temporal standard deviation (σT) of the ΔT series was used to assess estimation uncertainty.
Results
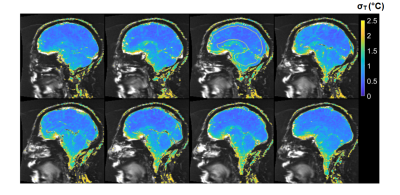
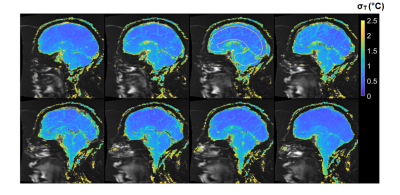
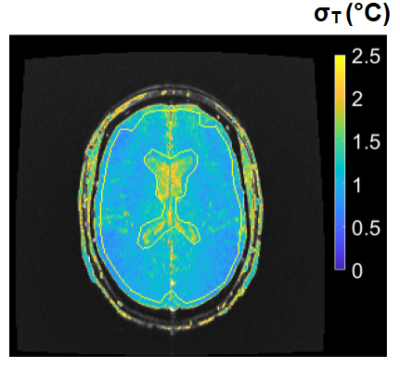
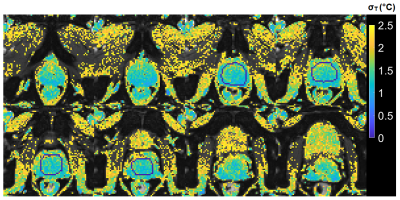
2D and 3D segmented EPI brain protocols resulted in excellent temperature precision (Figs. 1-4), with average σT of less than 1.1°C and 0.8°C respectively. 2D segmented EPI outperformed 2D single-echo GRE in terms of acquisition time as well as precision (Fig. 1). Average σT values ranging between 1.4°C and 1.6°C were observed in prostate across different subjects and protocols (Figs. 1 and 5).Discussion
The results presented in this abstract suggest that it is feasible to perform PRF thermometry in the brain and prostate at 0.55T, with σT within acceptable thresholds to monitor irreversible tissue damage. Frame rates achieved by our protocols are comparable with those used clinically at higher fields. In particular, the tested brain protocols provide a range of options in terms of scan time, spatial coverage, and acquisition strategy. Although the results of this initial investigation are encouraging, some key questions remain. First, it needs to be investigated whether B0 distortion resulting from longer EPI readouts is acceptable from a clinical perspective. Additionally, the possibility of exacerbated device-related artifacts in longer TE and ETL EPI acquisitions warrants further research. Future research will also focus on the investigation of other acquisition schemes such as spiral and multi-echo EPI for their use in MR thermometry.Low field MR-guided interventions offer some key advantages over high-field alternatives, such as reduced cost and improved safety of implantable and interventional devices, and reduced RF energy deposition. The application of 0.55T MRI for anatomical guidance is comparatively straightforward. The addition of PRF thermometry to the interventional MRI tools available at low field can help to expand the use of MRI as a modality for interventional guidance.
Acknowledgements
No acknowledgement found.References
1) Rieke, Viola, and Kim Butts Pauly. "MR thermometry." Journal of Magnetic Resonance Imaging 27.2 (2008): 376-390
2) Blackwell, James, et al. "Proton Resonance Frequency Shift Thermometry: A Review of Modern Clinical Practices." Journal of Magnetic Resonance Imaging (2020). doi: jmri.27446
3) Campbell-Washburn, Adrienne E., et al. "Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength MRI." Radiology 293.2 (2019): 384-393
4) Odéen, Henrik, et al. "Sampling strategies for subsampled segmented EPI PRF thermometry in MR guided high intensity focused ultrasound." Medical physics 41.9 (2014).doi: 10.1118/1.4892171
5) Majeed, Waqas, et al. “A Principal Component Analysis based Multi-baseline Phase Correction Method for PRF Thermometry.” Proceedings of ISMRM 27th Annual Meeting & Exhibition (2019): 3818
6) Tan, Jeremy, et al. "Motion compensation using principal component analysis and projection onto dipole fields for abdominal magnetic resonance thermometry." Magnetic Resonance in Medicine 81.1 (2019): 195-207
(*) MAGNETOM Free.Max is still under development and not yet commercially available. Its future availability cannot be guaranteed.
Figures
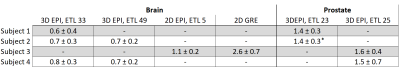
* The corresponding dynamic series consisted of 30 repetitions