1247
Rapid and Simultaneous Acquisition of T2-weighted and Fluid-attenuated Brain Images using a Spiral-ring Turbo Spin-echo Imaging1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Radiology & Medical Imaging, University of Virginia, Charlottesville, VA, United States
Synopsis
This study describes a new approach to obtain T2-weighted and fluid-attenuated inversion recovery (FLAIR) images simultaneously in a short scan time. In this technique, the Cartesian readout is replaced by an annular spiral-ring segmentation, and a 180°(y)-90°(x) preparation RF pulse at the end of the echo train is used for driven inversion, rather than a single 180° inversion pulse as used for conventional FLAIR. Both the T2-weighted and FLAIR images can be acquired in a single scan, with higher scan efficiency and similar image quality, when compared with Cartesian TSE and Cartesian FLAIR.
Introduction
T2-weighted and FLAIR pulse sequences are standard protocols for neuroimaging because of the high sensitivity for many brain lesions. The combination of these two techniques further improves lesion conspicuity, such as for multiple sclerosis1 and demyelination2. However, producing both of these image contrasts with high spatial resolution is time-consuming, typically taking 5-7 minutes, and sequential imaging may also be affected by motion artifacts and may require additional image registration for quantitative analysis. Takeo et al.3 reported use of the FASCINATE method to acquire T2-weighted and FLAIR images in a single acquisition, and the results showed the potential for this technique to replace the combination of conventional TSE and FLAIR sequences with a reduced scan time.Previously, a sampling strategy of annular spiral-ring (SPRING) segmentation has been incorporated into a 2D TSE signal generation module for rapid imaging4,5, and more recently we have introduced a SPRING TSE technique6 and its retraced in/out variant7 for high-quality T2-weighted brain images. In this work, we extended this idea to speed up acquisition time for combined T2-weighted/FLAIR imaging by replacing the Cartesian sampling in FASCINATE with a more efficient SPRING TSE in/out variant strategy.
Methods
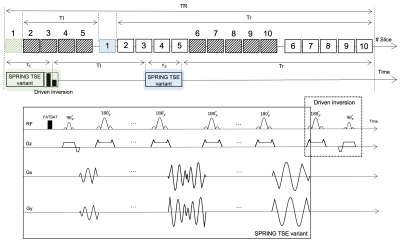
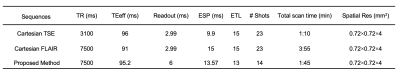
A simplified timing diagram of the pulse sequence is depicted in Figure 1. As described for FASCINATE, we used the same time-multiplexed multislice scheme to acquire both T2-weighted and FLAIR images. The boxes with stripes show slices for the T2-weighted acquisition, while the open boxes show slices for the FLAIR acquisition. The time interval between boxes with the same number is set to TI. A SPRING TSE in/out variant sampling scheme is used in each box for data acquisition, while a 180°(y)-90°(x) RF pair is applied only at the end of the echo train for boxes with stripes to achieve driven inversion. The longitudinal magnetization just before the T2-weighted (MT2w) and FLAIR (MFLAIR) sections can be expressed by the following equations:$$M_{T2w} = M_{0} \ast (1-e^{-(TR-\tau_{1}-TI)/T_{1}}) \qquad (1)$$ $$M_{FLAIR} = M_{0} - (M_{0} + \alpha \ast M_{T2w} \ast e^{-\tau_{1}/T_{2}}) \ast (e^{-TI/T_{1}}) \qquad (2)$$ where the coefficient $$$\alpha$$$ is introduced to express the efficiency of driven inversion, and $$$\tau_{1}$$$ is the interval between the first and last 90°(x) RF pulses. The effects of stimulated echoes (STEs) and magnetization transfer are ignored in these equations.Both phantom and in-vivo experiments were performed on a 3T scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) with a 32-channel head coil array. All images were obtained using Cartesian TSE, Cartesian FLAIR, and the proposed method. A TR of 7500ms was used for both Cartesian FLAIR and the proposed method, and a TR of 3100ms was used for Cartesian TSE. The echo spacing in the SPRING TSE variant sequence was 13.6ms, and the ETL was 13 ( $$$\tau_{1}$$$ = 183ms). To estimate the experimental value of the coefficient , several TI values were tested using a T1-T2 phantom. The total scan time was 1:10 min for Cartesian TSE, 3:55 min for Cartesian FLAIR, and 1:45 min for the proposed method. Other sequence parameters are given in Table 1.
Results and Discussion
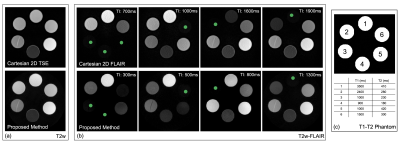
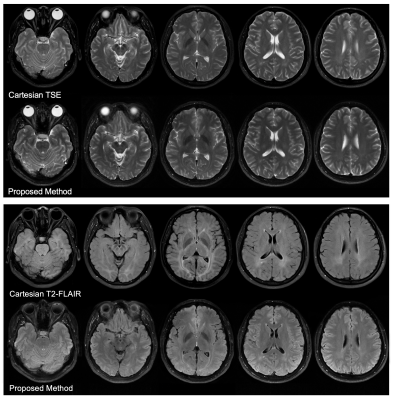
Figure 2 shows the effectiveness of the optimum TIs to null the signal in target voxels (green circles). The image contrast in some FLAIR images shows a slight difference between Cartesian and SPRING acquisitions, primarily because of a different signal model used in the proposed method that involves the T2 decay effect in Equation 2. The value of the coefficient $$$\alpha$$$ was estimated as $$$\alpha = 0.75$$$. Given the T1 and T2 values of CSF from Ref. 8 at 3T, the optimum TI was set to 1680ms for the proposed method.In-vivo images in Figure 3 show a comparison between Cartesian TSE and the T2-weighted acquisition of the proposed method, and between Cartesian FLAIR and the FLAIR acquisition of the proposed method. The T2-weighted images from the proposed method show good consistency with those from Cartesian TSE, in terms of image quality and image contrast. The FLAIR images from both of these methods show good CSF suppression and similar image quality, although there is a slight difference in the WM/GM contrast, mainly due to somewhat different imaging parameters used for these two methods, such as the echo time.
One drawback of our proposed method is that it may fail to nullify the CSF and suffer from flow artifacts in some imaging regions of fast CSF flow, due to the use of a thin slice-selective pulses. Another practical issue is the effect of stimulated echoes during the acquisition windows is not considered in the signal model, and increasing the ETL and the use of a reduced refocusing flip angle may further increase the contribution of the stimulated echoes. Future work will focus on a more complex signal model and the optimization of scan parameters.
Conclusion
A 2D brain imaging method for simultaneous acquisition of T2-weighted and FLAIR images, using a spiral-ring TSE sampling strategy combined with driven-inversion preparation pulses, was implemented and tested. The results show that the proposed method achieves T2-weighted and FLAIR images at 0.7 x 0.7 x 4 mm3 spatial resolution per slice in a single sequence with a 65% scan time reduction relative to sequential Cartesian acquisitions.Acknowledgements
No acknowledgement found.References
[1] Wiggermann V, Hernández-Torres E, Traboulsee A, Li D.K.B, Rauscher A. FLAIR2: a combination of FLAIR and T2 for improved MS lesion detection. AJNR Am J Neuroradiol 2016;37:259-65.
[2] Haller S, Kovari E, Herrmann FR, Cuvinciuc V, Tomm AM, Zulian GB, Lovblad KO, Giannakopoulos P, Bouras C: Do brain T2/FLAIR white matter hyperintensities correspond to myelin loss in normal aging? A radiologic–neuropathologic correlation study. Acta Neuropathol Commun 2013, 1:14.
[3] Kazuhiro Takeo, Akihiro Ishikawa, et al. FASCINATE: A Pulse Sequence for Simultaneous Acquisition of T2-Weighted and Fluid-Attenuated Images. Magnetic Resonance in Medicine 51:205-211, 2004.
[4] Block W, Pauly J, Nishimura D. RARE spiral T2-weighted imaging. Magn Reson Med. 1997;37:582–590
[5] Hennig J, Menza M, Barghoorn A, Riemenschneider B, Kroboth S, Zaitsev M. Spiral RARE with annular segmentation. Proceedings 27th Annual Meeting ISMRM, Montreal. 2019:4632.
[6] Wang Z, Allen S, Feng X, Mugler JP, Meyer CH. SPRING TSE: 2D T2-Weighted Brain Imaging using SPiral RING Turbo Spin-Echo. Proceedings 28th Annual Meeting ISMRM, Virtual Conference. 2020:3714.
[7] Wang Z, Allen S, Feng X, Mugler JP, Meyer CH. SPRING-RIO TSE: 2D T2-Weighted Turbo Spin-Echo Brain Imaging using SPiral RINGs with Retraced In/Out Trajectories. Proceedings 29th Annual Meeting ISMRM. Submitted.
[8] Bojorquez, J. Z., Bricq, S., Acquitter, C., Brunotte, F., Walker, P. M., & Lalande, A. (2017). What are normal relaxation times of tissues at 3 T? Magnetic Resonance Imaging, 35, 69-80.
Figures