1246
Simultaneous 3D T1 weighted, T2 weighted and FLAIR imaging using Serial Connection of Echo Train Acquisitions (SCETA) for Multi-contrast imaging
Naoyuki Takei1, Shohei Fujita2,3, Issei Fukunaga2, Mitsuharu Miyoshi1, Shigeki Aoki2, Suchandrima Banerjee4, and Tetsuya Wakayama1
1MR Applications and Workflow, GE Heatlcare, Tokyo, Japan, 2Juntendo University School of Medicine, Tokyo, Japan, 3The University of Tokyo Graduate School of Medicine, Tokyo, Japan, 4MR Applications and Workflow, GE Heatlcare, Menlo Park, CA, United States
1MR Applications and Workflow, GE Heatlcare, Tokyo, Japan, 2Juntendo University School of Medicine, Tokyo, Japan, 3The University of Tokyo Graduate School of Medicine, Tokyo, Japan, 4MR Applications and Workflow, GE Heatlcare, Menlo Park, CA, United States
Synopsis
To aim for accelerated brain routine MRI, a novel 3D multi-contrast imaging technique using the hybrid acquisition of FSE and GRE was proposed. The basic image contrasts commonly used in clinical practice such as T1 weighted, T2 weighted and FLAIR were acquired simultaneously. The comparison with conventional 3D imagings was performed in term of image contrast and scan time to demonstrate the proof of concept. The proposed technique potentially gives a new perspective of the use of 3D acquisition strategy.
Introduction
Multiparametric mapping and multi-contrast imaging have the potential to reduce MRI examination time by obtaining multiple contrast images in a single scan1-7. However, there are some challenges. The synthetic images suffer from partial volume effects. The generated FLAIR image has hyperintense artifacts1. The data acquisition methods that have been used in multi-contrast imaging are different from those used in routine clinical practice, resulting in the differences in image contrast and image artifact. GRE and EPI acquisitions which are often used in multi-contrast imaging for accelerated scan can acquire T1-weighted (T1w), T2-weighted (T2w) and FLAIR images, however which unfamiliar image contrast and artifact such as susceptibility artifact and image distortion can hinder clinical adoption. In this work, we have developed a new 3D multi-contrast imaging of the hybrid sequence using FSE and GRE to acquire T1w, T2w, and FLAIR simultaneously with similarity to commonly used image contrast and investigated its feasibility.Methods
Based on a 3D segmented acquisition, the serial connection of echo train acquisitions (SCETA) consists of two FSE blocks and one GRE in a TR on Figure.1a. The first FSE with variable refocusing flip angle builds T2 weighted contrast. Followed by the first inversion pulse to null fluid signal, the second variable refocused FSE provides T2 FLAIR contrast. Then the second inversion pulse to make T1 weighted contrast is followed by GRE. Echo train length (ETL) is the same in both FSE and GRE. In the SCETA, these two inversion pulses manipulate magnetization to obtain T1 and T2 weighted contrast nulling CSF in a TR (Figure.1b). The signal intensities in different tissues such as white matter (WM), gray matter (GM) and cerebrospinal fluid (CSF) simulated by Extended Phase Graphs8 with each ETL 200 in total 600 phase encodings indicate appropriate T1w, T2w and FLAIR image contrast. To compare with the conventional 3D acquisition of each T1w, T2w and FLAIR scan, healthy volunteer scan was performed under the IRB approval with the scan protocol in Table 1. A 3.0 T System (Discovery MR 750w, GE Healthcare, Waukesha, WI, U.S.A.) with 32 channel coils (MR instrument) was used. For SCETA scan parameter, the same variable refocusing flip angle of FSE was used in T2w and FLAIR. Sequential view ordering and centric view ordering are used in FSE and GRE, respectively. Spoiled-GRE (SPGR) is used in GRE with flip angle 10 degree. The same ETL of 200 is used to complete all the data acquisition at the same time. Parallel imaging technique, ARC9, was used with acceleration factor of 2 in all the scans.Results
Figure.2 and 3 showed the comparison result to each conventional scan with different slice positions in the transverse plane. The T1w, T2w and FLAIR image contrasts of SCETA appeared similar to those of the conventional scans. The scan time of SCETA was 3:00 and the total scan time of three conventional scans was 7:24 in the same spatial resolution and scan coverage. For the ROI measurements, the contrast between gray matter and white matter in SCETA was almost the same as that of the conventional scan in T2w and T1w. For FLAIR, the conventional FLAIR gives higher image contrast than SCETA. The contrast between CSF and white matter to measure CSF signal suppression was slightly higher in SCETA FLAIR than the conventional FLAIR and lower in SCETA T1w than conventional T1w.Discussions and conclusion
2D T1w, T2w, and FLAIR images has been used in many head routine protocols prioritizing imaging time and sacrificing spatial resolution along slice direction. In this abstract, we demonstrated that the multi-contrast imaging using the fast brain protocol of a spatial resolution of 0.8 x 0.8 x 4.0 mm3 and a TR 7500 ms provided comparable image contrast to the conventional methods whereas the scan time of the multi-contrast imaging was accelerated by a factor of approximately 2.4 than the total scan time of each conventional scan. Using SCETA, the 3D multi-contrast imaging that includes different image contrast generations into a TR would be a promising technique to obtain fast 3D images without compromising on imaging time, spatial resolution, and image contrast taking full advantage of the properties of 3D imaging. This technique not only reduces the actual scan time but also contributes to improve the efficiency of total head MRI scan, including prescan, scan workflow, and reading using the co-registered volumetric images.Acknowledgements
No acknowledgement found.References
- L.N. Tanenbaum, A.J. Tsiouris, A.N. Johnson, et al. Synthetic MRI for clinical neuroimaging: results of the magnetic resonance image compilation (MAGiC) prospective, multicenter, multireader trial. AJNR Am. J. Neuroradiol., 38 (June (6)) (2017), pp. 1103-1110.
- Ma D, Gulani V, Seiberlich
N, et al. Magnetic Resonance Fingerprinting. Nature. 2013;495(7440):187-192.
- Skare S, Sprenger T, Norbeck O. A 1-minute full brain MR exam using a multicontrast EPI sequence. Magn Reson Med 2018;79:3045– 3054.
- Chen Y, Liu S, Wang Y, Kang Y, Haacke EM. STrategically Acquired Gradient Echo (STAGE) imaging, part I: creating enhanced T1 contrast and standardized sus- ceptibility weighted imaging and quantitative susceptibility mapping. Magn Reson Imaging 2018;46:130–9.
- Kvernby S, Warntjes MJ, Haraldsson H, Carlhäll CJ, Engvall J, Ebbers T. J Cardiovasc Magn Reson. 2014 Dec 20;16:102. Simultaneous three-dimensional myocardial T1 and T2 mapping in one breath hold with 3D-QALAS.
- Hwang et al, Proc. Intl. Soc. Mag. Reson. Med. 18 (2019), 5627.
- Wang F, Dong Z, Reese TG, Bilgic B, Katherine Manhard M, Chen J, Polimeni JR, Wald LL, Setsompop K. Magn Reson Med. 2019 Jun;81(6):3599-3615.
- Weigel M. Extended phase graphs: Dephasing, RF pulses, and echoes -‐pure and simple. J. Magn. Reson. Imaging 2015;41:266–295.
- Brau AC, Beatty PJ, Skare S, Bammer R. Comparison of reconstruction accuracy and efficiency among autocalibrating data‐driven parallel imaging methods. Magn Reson Med 2008; 59: 382– 395.
Figures
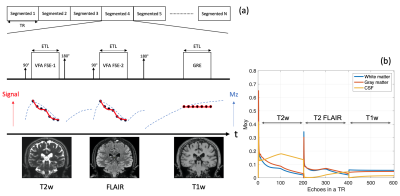
Fig.1. (a) A schematic pulse sequence diagram of
SCETA (Serial Connection of Echo Train acquisition) to acquire T1w, T2w and
FLAIR using the hybrid acquisition of FSE and GRE. (b) Echo signal of white
matter, gray matter and CSF builds three imaging contrasts in a TR.
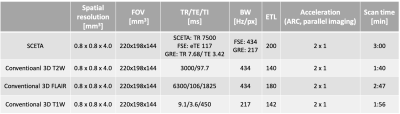
Table 1 scan
parameter of SCETA and conventional 3D scans
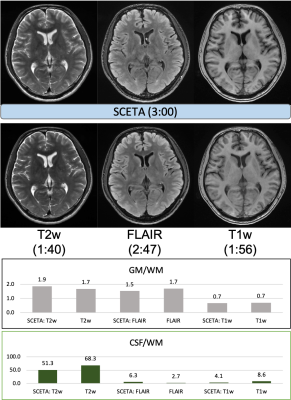
Fig. 2. A
comparsion result with conventional 3D scan of T2w, FLAIR and T1w. SCETA
reduces scan time by a factor of approximately 2.4 while maintaining similar
image appearance to conventional scan.
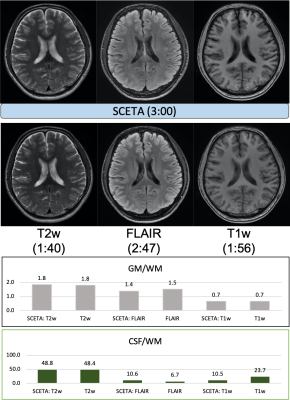
Fig.3.
Different slice position from Fig.2. Similar results as in Fig.2 are obtained.