1225
A pilot study of socially deprivated brain alterations using fMRI and dMRI in domestic dogs1State Key Laboratory of Brain and Cognitive Science, Institute of Biophysics, Chinese Academy of Sciences, BeiJing, China, 2University of Chinese Academy of Sciences, Beijing, China, 3State Key Laboratory of Molecular Developmental Biology, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China, 4CAS Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Beijing, China
Synopsis
Social interactions, like sleep, is the basic needs for humans and animals, its neural mechanism is not fully understood. Domestic dog has similar emotional and social processing with humans, it would be a promising animal model for social interaction. In this study, 3 beagles were social deprived (SD) 4-week, and performed resting-state fMRI and diffusion MRI. The functional connectivity and diffusion metrics were compared between SD and wild type dogs. We found the functional connectivity strengthened within prefrontal cortex and weakened between prefrontal cortex with visual and auditory cortex in SD. Demyelination observed in frontal, temporal, and insula regions.
Introduction
Social interactions, like food consumption and sleep, are proposed to be the basic needs of humans and animals.1 However, due to the violation of ethics for social deprivation in human subjects and the lack of animal models socially communicating with humans (such as rats),2 its neural mechanism is still not fully understood. As a species with a long history of evolution to live and work with human beings, domestic canine has similar emotional and social processing with humans.3 A previous study showed the alteration of brain state in the prefrontal, parietal, and temporal regions.4 If we socially deprive domestic dogs, will their brain physiologic change?Resting-state functional MRI (rs-fMRI) is sensitive to brain function synchronization, which could reflect the alterative functional correlations between brain regions.5 Diffusion MRI (dMRI) can measure the microstructure integrity of neuronal fibers through fractional anisotropy (FA), radial diffusivity (RD), mean diffusivity (MD), and axial diffusivity (AD).6,7 rs-fMRI and dMRI would provide an opportunity to study the changes in brain structure and function of domestic dogs with different social experiences (social isolation and social deprivation), which provides insight into the mechanism of canine on social and emotional and cognitive functions. In this study, 3 social deprivation and 14 wild type Beagles have performed MRI scans.
Methods
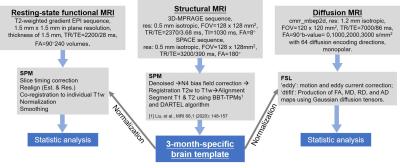
Data acquisition: All dogs were male and divided into social deprivation (SD) group (3 Beagles, age 93.67 ± 0.47 days, weight 4.67 ± 0.94 kg) and wild type (WT) group (14 Beagles, age 96.21 ± 10.28 days, weight 4.42 ± 1.33 kg). For social deprivation experiments, 3 males were selected from 3 litters naturally weaning at postnatal day 51. They were reared together in a cage until day 65 and then were housed individually for 28 days. All the experimental protocols were approved by the local institute Animal Care and Committee. Seventeen Beagles were scanned at 3T MRI scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) with a home-made 4-channel Tx/Rx RF coil to obtain high quality structural magnetic resonance imaging (sMRI), rs-fMRI, and dMRI. All parameters and image processing details were summarized in Fig.1. Each dog was anesthetized similar to our previous study.8Image analysis: A 3-month-specific Beagle brain template and tissue probability maps (TPM) were created following our previous pipelines.8 For functional datasets, the pre-processed rs-fMRI images were detrended, regressed out the mean signal from white matter and cerebral spinal fluid (CSF), and filtered by temporal band-pass of 0.008-0.1 Hz. Eighty-six regions of interest (ROIs) were chosen according to the canine brain atlas9 for rsfMRI analysis and diffusion statistic. The Pearson correlation coefficients were calculated between the time course of ROIs. Brain connectivity strength differences in ROIs were assessed a strict method, in which the minimum and maximum R-values of the SD were compared between that of WT to find the specific brain regions with significant differences due to a small sample size of SD dogs. For diffusion datasets, the original diffusion images were preprocessed using FSL, including motion and eddy-current corrected. FA, MD, RD, and AD maps were obtained using ‘dtifit’ algorithms. The 86 ROIs were manually divided into eight areas according to the location in the brain: frontal, parietal, temporal, occipital, cerebellum, insula, and cingulate, totaling 53 ROIs. All remaining ROIs were divided into the eighth area. The diffusion maps of the first 7 areas in cortical gray matter and white matter were differently compared between the SD and WT group using a two-sample unpaired t-test.
Results
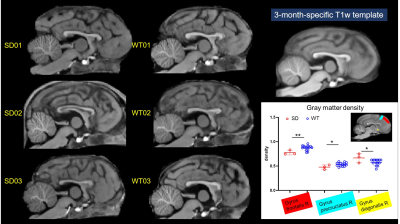
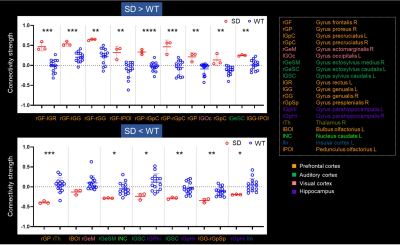
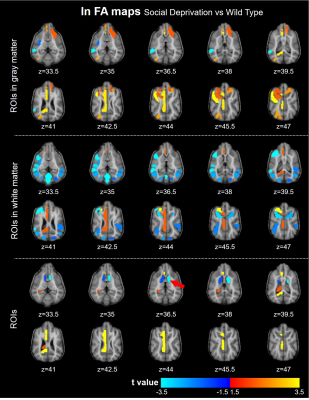
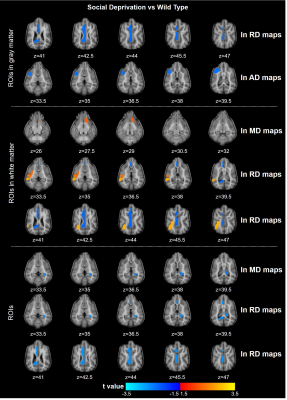
Fig.2 displays the structural images from 3 SD and 3 WT dogs. There was no significant difference in corpus callosum, cingulate, and the other brain structure. The gray matter density of the SD decreased only in the frontal lobe. Compared with WT, the functional connectivity (FC) between ROI within the prefrontal cortex (PFC) in SD was overall enhanced, and the FC between lGG and rGpSp was weakened. The FC between the rGP and the lGOc enhanced, the FC between lBOl and rGeM weakened, the FC between the rGP and the right thalamus weakened. The weaken FCs between the auditory cortex and the parahippocampal gyrus, caudate nucleus were observed. The FC between the hippocampus and the insula weakened (Fig.3). The diffusion metrics showed that the brain of the socially deprived dogs in the frontal, temporal lobes, and cerebellum had significantly decreased FA values and increased RD values, especially ROIs in the white matter in Fig.4 and Fig.5.Discussion and Conclusion
The FCs within the frontal cortex were strengthened and the FCs between PFC with the visual and auditory cortex were weakened after 4 weeks socially deprived of Beagle dogs. And the frontal and insula region demyelinated after socially deprived.2,4,10 These results were consistent with human and children studies. The canine would be a promising experimental animal for emotion study. The MRI could detect the brain slight alteration after social deprivation. It is feasible to study the mechanisms of social interaction and emotion mechanism in-vivo using MRI in domestic dogs.Acknowledgements
This work was supported in part by the Ministry of Science and Technology of China (2019YFA0707100, 2020AAA0105601), the National Natural Science Foundation of China (31730039, 31830036, and 31921002), and the Chinese Academy of Sciences Strategic Priority Research Program B grants (XDB32010300, XDBS1020100, and XDB13041000).References
1. Baumeister RF, Leary MR. The need Belong: Desire for interpersonal attachments as a fundamental human motivation. Psychol Bull. 1995;117(3):497-529.
2. Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434-445.
3. Müller CA, Schmitt K, Barber ALA, Huber L. Dogs can discriminate emotional expressions of human faces. Curr Biol. 2015;25(5):601-605.
4. McLaughlin KA, Sheridan MA, Winter W, Fox NA, Zeanah CH, Nelson CA. Widespread reductions in cortical thickness following severe early-life deprivation: A neurodevelopmental pathway to attention-deficit/hyperactivity disorder. Biol Psychiatry. 2014;76(8):629-638.
5. Lv H, Wang Z, Tong E, et al. Resting-state functional MRI: Everything that nonexperts have always wanted to know. Am J Neuroradiol. 2018;39(8):1390-1399.
6. Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005;26(4):1164-1173.
7. Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4(6):469-480.
8. Liu X, Tian R, Zuo Z, et al. A high-resolution MRI brain template for adult Beagle. Magn Reson Imaging. 2020;68(1):148-157.
9. Czeibert K, Andics A, Petneházy Ö, Kubinyi E. A detailed canine brain label map for neuroimaging analysis. Biol Futur. 2019;70(2):112-120.
10. Liu J, Dietz K, Deloyht JM, et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci. 2012;15(12):1621-1623.
Figures