1224
Characterization of cardiac function in early-stage pulmonary arterial hypertension in asymptomatic BMPR2 mutant rats by MRI and 31P MRS1IHU LIRYC, Bordeaux, France, 2INSERM U1045, CRCTB, Bordeaux, France, 3INSERM UMR_S 999, Université Paris–Saclay, Le Kremlin Bicêtre, France
Synopsis
The transgenic rat line studied has a monoallelic mutation of Bmpr2 which is a model of heritable pulmonary arterial hypertension. The purpose of this study is to evaluate cardiac differences between wild type and asymptomatic mutated rats by cardiac MRI and 31P MRS. Transgenic rats showed a decrease in end-diastolic and end-systolic volumes of ventricles leading to a reduced stroke volume. These results indicate a significant decrease in cardiac output but without ventricular hypertrophy (constant wall thickness) and no significant variation of ejection fraction. The cardiac energy balance (phosphocreatine/ATPβ) remained unaltered in Bmpr2 rats at rest and post-injection of dobutamine.
Introduction
Advanced stage of pulmonary arterial hypertension (PAH) is characterized by an increase in the pulmonary arterial pressure along with right ventricular failure leading to the patient death1. In heritable cases, PAH is mostly associated to monoallelic mutations in Bone Morphogenic Protein Receptor 2 (Bpmr2) gene. The first rat model with a monoallelic mutation of Bmpr2 (deletion of 71bp in exon 1 leading to loss of the initiation codon) showed a similar penetrance of PAH as the one observed in humans (~20%): 16.7% at 6-month-old and 27.8% at 1-year-old2. The aim of this study is to characterize early cardiac modifications in asymptomatic mutated rats by magnetic resonance imaging (MRI) and 31P magnetic resonance spectroscopy (31P MRS).Methods
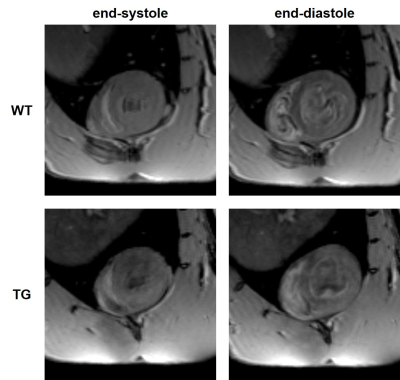
Two groups of 3-month-old male Sprague Dawley rats were used for MR experiments: transgenic (TG, bmpr2 monoallelic mutation, n=12) and wild type (WT, non-mutated brothers, n=11). MR acquisitions were performed on a 9.4T Biospec MRI (Bruker, Wissembourg, France) of 20 cm aperture. A transmit- quadrature coil coupled with phase array coil in reception was used for MRI and a 1H/31P surface coil for spectroscopy. All animal experimental procedures were performed in accordance with the recommendations of the European Union (2010/63/EU) for care and use of laboratory animals and conformed to the ethical guidelines of the French Ministry of Agriculture and Forests (Animal Health and Protection Veterinary Service). The protocol was approved by the ethics committee under the reference “APAFIS#23880-2020013112499366-v4”. Animals were anesthetized with 4% isoflurane then maintained at 2% (1L.min-1 mixed in air) during acquisitions. The physiological parameters were monitored inside the magnet with ECG electrodes taped on the forepaws and a respiration pad placed against the abdomen.Several scout images were acquired to determine the short axis view of the rat heart (Fig.1). Short-axis cine FLASH was acquired with the following parameters: TE/TR= 1.60/80ms; flip angle= 15°; 20 contiguous slices; in-plane resolution= 300x300µm2; slice thickness= 1mm; 10 frames per cardiac cycle; 5 averages; cardiac and respiratory triggering. These MR images were used to assess: (i) end-diastolic (ED) and end-systolic (ES) volumes of left ventricle (LV) and right ventricle (RV); (ii) ejection fraction (FE) of both ventricles and (iii) wall thickness of mid-LV, mid-septum and mid-RV at end-diastolic stage. Images were analyzed on Horos (LGPL-3.0) with MRHeart plugin3.
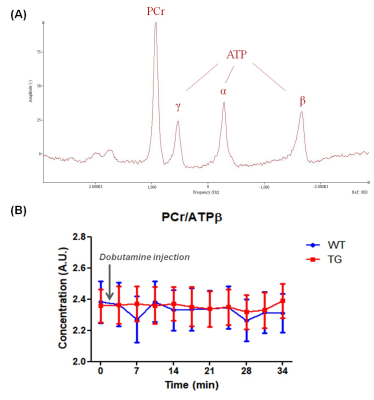
Localized 31P spectra were obtained on ventricles by outer volume suppression method4: 6 saturation bands were surrounding the volume of interest (10x10x14mm3) followed by a non-selective pulse of 200µs (α= 90°; TR= 1.5s; bandwidth= 8KHz; 4096 points; 64 averages) triggered onto the respiration and cardiac cycle. Beforehand, a localized shimming on the heart was performed with the use of the 1H coil, resulting in a proton line width of approximately 65Hz. Each spectrum was obtained in 3min12s over a duration of 35min, first at rest and after injection of cardiac β-adrenergic agonist (intravenous injection of 10µg/kg of dobutamine through caudal vein). Phosphocreatine (PCr) and ATPβ were quantified by AMARES (apodization of 20Hz) (jMRUI 6.0) to assess the cardiac energy balance (Fig.5A). Groups were compared by nonparametric t test Mann-Whitney with *p<0.05; **p<0.01; ***p<0.001 (GraphPad Prism 8.0).
Results
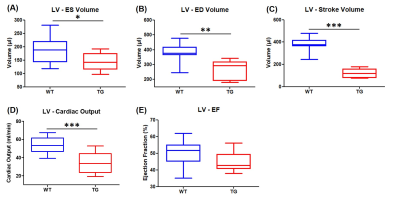
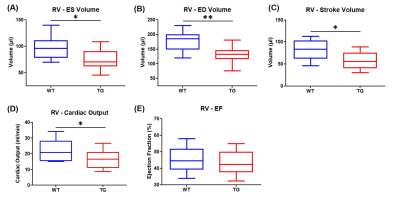
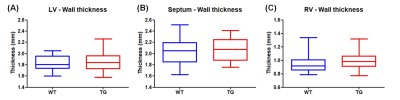
Volumetric measurements showed a significant decrease in end-systolic and end-diastolic volumes of LV in TG rats compared to WT rats: -22.7% and -28.9% respectively (Fig.2A&2B). Similarly, a reduced RV volume was measured in TG group in comparison to WT group: -23.1% at end-systolic stage and 25.2% at end-diastolic stage (Fig.3A&3B). Thereby, a significantly reduced stroke volume was calculated in both ventricles of TG rats: -35.1% for LV (Fig.2C) and -28.1% for RV (Fig.3C). These decreases led to a significant decrease in cardiac output in TG group compared to WT group: -19.0% for LV (Fig.2D) and -6.6% for RV (Fig.3D). No significant difference in ejection fraction was found between both groups for LV (WT=50.2±2.3%; TG=44.9±1.6%) and RV (WT=45.3±2.1%; TG=43.3±2.1%) (Fig.2E&Fig.3E). In addition, no difference of wall thickness was measured in mid-LV, mid-septum and mid-RV between the groups (Fig.4).The ratio of PCr/ATPβ did not show any difference at rest between the groups (Fig.5B). The injection of dobutamine led to a 16% increase in heart rate (from ~290bpm to ~345bpm) in both groups with a progressive return to the basal value after ~15min. Despite this heart rate elevation, no significant variation of the ratio PCr/ATPβ was measured after injection of dobutamine in both groups (Fig.5B).
Conclusion
Transgenic rat model of familial PAH showed reduced end-diastolic and end-systolic volumes of ventricles leading to a reduced stroke volume. These results indicate a decrease in cardiac output without ventricular hypertrophy (constant wall thickness) and no significant variation of ejection fraction, thus resembling heart failure with preserved ejection fraction. Cardiac energy balance remained unchanged in Bmpr2 rats compared to control rats, at rest and following β-adrenergic stimulation. Cardiac output modifications appear as an early adaptation prior to established PAH. Further studies will be conducted in 6-month-old rats in purpose to evaluate if functional cardiac MRI and 31P MRS can provide noninvasive prognosis of familial PAH.Acknowledgements
This study received financial support from the French Government as part of the “Investments of the Future” program managed by the National Research Agency (ANR), grant reference "ANR-10-IAHU-04". This project also received financial support from the French Federation of Cardiology (FFC), grant reference "FFC Quesson".References
1. Machado, R. D. et al. Genetics and Genomics of Pulmonary Arterial Hypertension. J. Am. Coll. Cardiol. 54, S32–S42 (2009).
2. Hautefort, A. et al. Bmpr2 Mutant Rats Develop Pulmonary and Cardiac Characteristics of Pulmonary Arterial Hypertension. Circulation 139, 932–948 (2019).
3. Szychta, W., Werys, K., Barczuk-Falęcka, M., Postuła, M. & Małek, Ł. Validation of performance of free of charge plugin for the open-source platform to perform cardiac segmentation in magnetic resonance imaging. Heart Beat J. 3, 83–89 (2019).
4. Deschodt-Arsac, V. et al. Energy Deregulation Precedes Alteration in Heart Energy Balance in Young Spontaneously Hypertensive Rats: A Non Invasive In Vivo 31P-MR Spectroscopy Follow-Up Study. PLOS ONE 11, e0162677 (2016).
Figures