1222
Multiparametric functional magnetic resonance imaging for longitudinal evaluation of liver regeneration after 70% hepatectomy in a rat model1Tianjin First Central Hospital, Tianjin, China, 2MR Collaboration, Siemens Healthcare Ltd., Beijing, China, 3MR Application Development, Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
This study investigated the feasibility of multiparametric magnetic resonance imaging (MRI) (IVIM, DKI, BOLD) to evaluate the liver regeneration process after 70% hepatectomy in rats. MRI examination and pathologic samples were performed at multiple time-points. The results showed that D, D*, FP, MD, MK, and T2* can be used to monitor the microstructural changes after surgery, and D* and MK were significantly correlated with proliferative indexes of Ki - 67 and hepatocyte size. This suggests that multiparametric MRI can aid in noninvasive radiologic monitoring of liver regeneration and intrinsic microstructure evaluation
Introduction and purpose
Partial hepatectomy (PH) is the primary treatment method for liver tumors. Sufficient residual liver function is critical for surgical planning and patient outcomes [1]. Increasing liver volumes are used to evaluate regeneration activity but might not reflect true increases in functional liver tissue. Multiparametric magnetic resonance imaging (MRI) has been increasingly used to evaluate liver microstructural conditions [2-3]. Previous studies have confirmed the feasibility of using conventional diffusion-weighted imaging (DWI), diffusion kurtosis imaging (DKI), and T1 relaxation times to evaluate liver regeneration microstructure [4-6]. After PH, the blood supply changed immediately [7], and the size and structural complexity of hepatocytes changed gradually during regeneration [8]. This study was conducted to investigate the value of intravoxel incoherent motion (IVIM), diffusion kurtosis imaging (DKI), and blood oxygen level-dependent (BOLD) MRI in assessing liver regeneration and microstructural changes in a rat model after 70% hepatectomy.Methods
Thirty-four rats were used for the 70% hepatectomy model, and ten were randomly selected for longitudinal MRI examination. All the MRI examinations were conducted on a 3T MR scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) with an 8-channel animal coil (Chenguang, Shanghai, China). IVIM, DKI, and BOLD MRI were performed before and 0 (within two hours), 1, 2, 3, 5, 7, 14, and 21 days after surgery. The other 24 rats were selected and sacrificed for histopathologic evaluation, and three were randomly selected at each time-point (except day 0). Liver BOLD MRI was acquired using a multi-echo gradient echo sequence with repetition time (TR) = 400 ms and 6 echo times (TEs) in the range of 2.46 to 15.01 ms, with an echo interval of 2.51 ms. A single-shot echo-planar DWI sequence was used to acquire DWI data. Thirteen b-values of 0, 10, 20, 30, 50, 100, 200, 300, 500, 800, 1000, 1500, and 2000 s/mm2 were obtained in three diffusion gradient directions. BOLD-derived parameter (T2*) parametric maps were generated inline after data acquisition using MapIt software (Siemens Healthcare, Erlangen, Germany). Ten b-values (0, 10, 20, 30, 50, 100, 200, 300, 500, and 800 s/mm2) were selected for the postprocessing of the IVIM model, and five b-values (0, 500, 1000, 1500, and 2000 s/mm2) were chosen for the DKI model. IVIM-derived parameters (D, diffusion coefficient; D*, pseudo-diffusion coefficient) and DKI-derived parameters (MD, mean diffusivity; MK, mean kurtosis) were generated using a prototype post-processing software (MR Body Diffusion Toolbox, Siemens Healthcare, Erlangen, Germany). All the quantitative parameters of liver parenchyma were measured to compare among the different time-points. Radiologic-pathologic correlations were also evaluated.Results
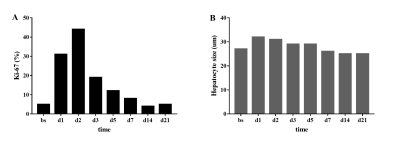
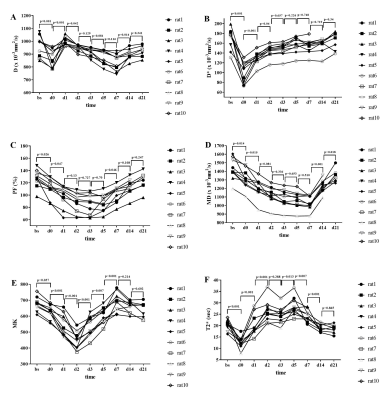
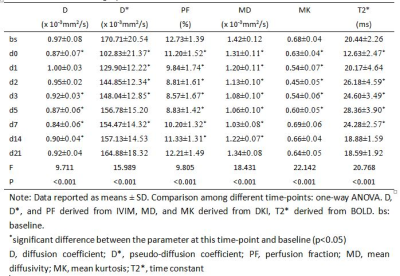
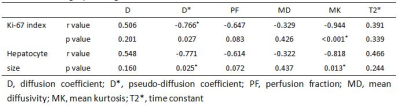
During the MRI examination on days 14 and 21 after surgery, one rat and two rats, respectively, died due to anesthesia. The Ki-67 proliferative indices increased rapidly after surgery and peaked at day 2, and then decreased rapidly at day 3, gradually reaching baseline at day 14 (Figure 1A). Hepatocyte sizes increased slightly 1 day after surgery and decreased gradually to baseline at day 7 (Figure 1B). All MR parameters significantly decreased within 2 hours after surgery, changed regularly, and finally returned to baseline 14 or 21 days after surgery (all p< 0.05, Table 1, Figure 2). The IVIM-derived parameter, D*, and the DKI-derived parameter, MK, were significantly correlated with the Ki-67 indices and hepatocyte sizes (Table 2).Discussion
IVIM, DKI, and BOLD-derived parameters changed gradually after 70% hepatectomy. D*, perfusion fraction (PF), and T2* values can reflect blood supply changes, D and MD values can reflect changes in water molecule movements, and MK can reflect complex liver tissue changes. Moreover, DP and MK were significantly correlated with hepatocyte regeneration indices and sizes, indicating that MD and MK could be used as non-invasive indices to reflect liver regeneration activity. The ability of MRI to assess liver regeneration could be valuable for clinical management.Conclusion
IVIM, DKI, and BOLD can monitor the regenerative process and intrinsic microstructural changes of rat livers after 70% hepatectomies. D* and MK values are particularly useful in monitoring liver regenerative activity.Acknowledgements
This work was supported by the National Natural Science Foundation for Young Scientists of China (81901710).References
1. Vennarecci G, Laurenzi A, Levi Sandri GB, Busi Rizzi E, Cristofaro M, Montalbano M, Piselli P, Andreoli A, D'Offizi G, Ettorre GM. The ALPPS procedure for hepatocellular carcinoma. Eur J Surg Oncol. 2014;40(8):982-988
2. Yang L, Rao S, Wang W, et al. Staging liver fibrosis with DWI: is there an added value for diffusion kurtosis imaging? Eur Radiol. 2018;28(7):3041-3049.
3. Cao L, Chen J, Duan T, Wang M, Jiang H, Wei Y, Xia C, Zhou X, Yan X, Song B. Diffusion kurtosis imaging (DKI) of hepatocellular carcinoma: correlation with microvascular invasion and histologic grade. Quant Imaging Med Surg. 2019;9(4):590-602.
4. Eberhardt C, Wurnig MC, Wirsching A, et al. Prediction of small for size syndrome after extended hepatectomy: Tissue characterization by relaxometry, diffusion weighted magnetic resonance imaging and magnetization transfer. PLoS One. 2018;13(2):e0192847.
5. Sheng RF, Yang L, Jin KP, et al. Assessment of liver regeneration after associating liver partition and portal vein ligation for staged hepatectomy: a comparative study with portal vein ligation. HPB (Oxford). 2018;20(4):305-312.
6. Sheng RF, Wang HQ, Jin KP, et al. Histogram analyses of diffusion kurtosis indices and apparent diffusion coefficient in assessing liver regeneration after ALPPS and a comparative study with portal vein ligation. J Magn Reson Imaging. 2018;47(3):729-736.
7. Court FG, Wemyss-Holden SA, Dennison AR, et al. The mystery of liver regeneration[J]. Br J Surg. 2002;89(9):1089-1095.
8. Miyaoka Y, Ebato K, Kato H, et al. Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration[J]. Curr Biol. 2012;22(13):1166-1175.
Figures