1219
Thalamic reticular nucleus injury as the cause of thalamocortical dysrhythmia in mild traumatic brain injury: a rodent model DTI study1Translational Imaging Research Center, Taipei Medical University Hospital, Taipei, Taiwan, 2Department of Medical Imaging, Taipei Medical University Hospital, Taipei, Taiwan, 3Neuroscience Research Center, Taipei Medical University, Taipei, Taiwan
Synopsis
Thalamocortical dysrhythmia (TCD) has been implicated in the neuropsychiatric disorders in patients following mild traumatic brain injury (mTBI). We investigated the time-course diffusion tensor changes in the rodent brain up to 35 days using a controlled closed head injury model. Significant and persistent elevation of fractional anisotropy (FA) and reduced radial and mean diffusivities were found at the boundary of bilateral thalami where high shear stress was expected, suggesting that disinhibition of inhibitory circuits from the thalamic reticular nucleus (TRN) may play a role in TCD.
Introduction
The post-concussive syndrome (PCS) in mild traumatic brain injury (mTBI) has been implicated to thalamocortical dysrhythmia (TCD), a hyperpolarized thalamocortical transmission with persisting cross-frequency coupling between theta and gamma activity, in several studies[1-3]. Although most patients recover, a substantial minority suffers chronic behavioral and cognitive impairment. The pathomechanisms underlying PCS may be attributed to the dysfunctionality of regulatory and inhibitory circuity in the thalamocortical network involving the GABAergic inhibitory thalamic reticular nucleus (TRN)[4] by shearing force during the impact[5]. Although numerous diffusion tensor imaging (DTI) studies have explored axonal injury after mTBI[ 6,7], the effects of mTBI on TRN are not well assessed. In the present study, we proposed to examine the microstructural changes in cerebral cortex, white matter(WM), thalamus and TRN by DTI in a mTBI rodent model, and to characterize the evolution of axonal injury following mTBI.Materials and Methods
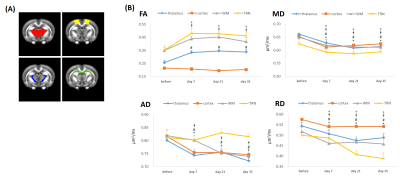
This study was approved by the local institutional animal care and use committee. Five male rats subjected to well-controlled weight-drop injury model. A 600-g weight was dropped from a height of 1 m through a stainless steel tube on the left sensorimotor cortex. Animals received 2 impacts with a 1-hour interval[8]. Longitudinal MR imaging was performed before, at 7 days, 21 days and 35 days after the impact using a 7T system. DTI images were acquired with the following parameters: TR/TE=3000/28 ms, Δ/δ =15/5ms, 30 non-collinear gradient directions, 16 slices of 1.0 mm thickness and in-plane resolution of 0.16x0.16 mm2. DTI metrics such as fractional anisotropy(FA), mean diffusivity (MD), axial diffusivity(AD), and radial diffusivity(RD) were derived using MRtrix and standard FSL pipeline. For statistical comparison between rats, each rat brain volume was co-registered and normalized to a template rat atlas[9]. The voxel-wise statistics for DTI-derived maps were conducted by using SPM12. Brain areas with uncorrected P<0.001 and a cluster size of more than 10 voxels was considered statistically significant and was superimposed on the group-averaged FA maps generated from 5 pre-impact rats for better visualization. This was subsequently followed by region-of-interest (ROI) analysis (cortex, thalamus, WM and TRN, Figure 3A) over the regions that showed significant differences on the t-maps. Paired-sample t-test was applied to examine the significance between the pre-impact and follow-up data.Results
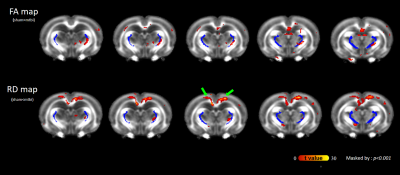
As compared with pre-impact, clusters with significantly increased FA at the boundaries of the bilateral thalami were observed after impact (Figure 1; green arrows), supporting the axon and/or myelin injuries of TRN (Figure 1; dark blue). Figure 2 shows the evolution of FA changes after impact. At day 7, FA changes were seen only in unilateral TRN with a lesser extent (green arrows). At day 21, FA changes increased in extent (yellow arrows) and some clusters of FA changes were noted on the contralateral side near TRN (black arrows). Finally, FA changes with a larger extent were shown in/near bilateral TRN at day 35, albeit the unilateral impact. For qualitative comparison, FA significantly increased by 25.7±5.7% in thalamus(p<0.01), 24.4±3.4% in WM(p<0.05) and 28.5±7.8% in TRN(p<0.05) at day 7, respectively, as well as remained increased with time (Figure 3B; FA). Contrary to FA, MD, AD and RD values showed significantly decreases by approximately an average of -7.0% in thalamus, WM, TRN and cortex at day 7, respectively, as well as showed tendencies to decline (Figure 3B). It is worth noting that significantly increased FA were not found in cortex (Figure 3B; FA), and significantly decreased AD were also not seen in TRN (Figure 3B; AD). Figure 4 demonstrates that there were no FA changes in cortex region at day 7 whereas RD changes were observed. The no FA changes in cortex was possibly due to the comparable reductions in AD and RD (-7.8% vs -6.5%, respectively, p>0.05).Discussions
Our results indicated that significantly elevated FA and reduced RD and MD within TRN during the first 35 days after impact as compared to similar patterns of evolutions in DTI metrics in thalamus, WM and cortex. The finding of altered DTI metrics in TRN may is consistent with the previous shear model showing that the injuries are distributed in the grey white matter junctions around the thalami that may encompass TRN[5]. Damages in GABAergic TRN would likely result in the reduction in inhibitory effects on the thalamus, therefore, increase thalamocortical activities. Conversely, damages in thalamic neurons may account for the decrease in neuronal activity in the thalamocortical pathways[10]. TCD could be a consequence of these microstructural damages. Reduced MD might be the result of CHI-induced cytotoxic edema and increased intracellular water content restricts the molecular motion of water that leads to an increase of FA[11]. High FA in combination with low RD is hypothesized to reflect inflammation or cytotoxic edema[12]. Interestingly, a discrepancy between FA and RD changes in cortex region suggests that the main quantitative metric FA may be less sensitive to uncover significant abnormalities in grey matter structure since FA is an interplay between AD and RD. FA, MD, AD, and RD are mutually related, and these metrics should be explored in an effort to improve the sensitivity of DTI to mTBI.Conclusions
Characterization of TRN injury can better understand the underpinnings of diverse neuropsychiatric symptoms following mTBI.Acknowledgements
No acknowledgement found.References
1.Tang L, Ge Y, Sodickson DK, et al. Thalamic resting-state functional networks: disruption in patients with mild traumatic brain injury. Radiology. 2011;260(3):831-840.
2.Chen C-J, Wu C-H, Liao Y-P, et al. Working memory in patients with mild traumatic brain injury: functional MR imaging analysis. Radiology. 2012;264(3):844-851.
3.Fakhran S, Yaeger K, Collins M, Alhilali L. Sex differences in white matter abnormalities after mild traumatic brain injury: localization and correlation with outcome. Radiology. 2014;272(3):815-823.
4.Min B-K. A thalamic reticular networking model of consciousness. Theoretical Biology and Medical Modelling. 2010;7(1):1-18.
5.Mendez CV, Hurley RA, Lassonde M, Zhang L, Taber KH. Mild traumatic brain injury: neuroimaging of sports-related concussion. The Journal of Neuropsychiatry and Clinical Neurosciences. 2005;17(3):297-303.
6.Borja MJ, Chung S, Lui YW. Diffusion MR imaging in mild traumatic brain injury. Neuroimaging Clinics. 2018;28(1):117-126.
7.Smith LG, Milliron E, Ho M-L, et al. Advanced neuroimaging in traumatic brain injury: an overview. Neurosurgical Focus. 2019;47(6):E17.
8.Kao Y-C, Lui Y, Lu C-F, Chen H-L, Hsieh B-Y, Chen C-Y. Behavioral and structural effects of single and repeat closed-head injury. American Journal of Neuroradiology. 2019;40(4):601-608.
9.Straathof M, Blezer EL, van Heijningen C, et al. Structural and functional MRI of altered brain development in a novel adolescent rat model of quinpirole-induced compulsive checking behavior. European Neuropsychopharmacology. 2020.
10.Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain research reviews. 2004;46(1):1-31.
11.Liu H-S, Chou M-C, Chung H-W, et al. Potential Long-term Effects of MDMA on the Basal Ganglia–Thalamocortical Circuit: A Proton MR Spectroscopy and Diffusion-Tensor Imaging Study. Radiology. 2011;260(2):531-540.
12. Hoogenboom WS, Rubin TG, Ye K, et al. Diffusion tensor imaging of the evolving response to mild traumatic brain injury in rats. Journal of Experimental Neuroscience. 2019;13:1179069519858627
Figures
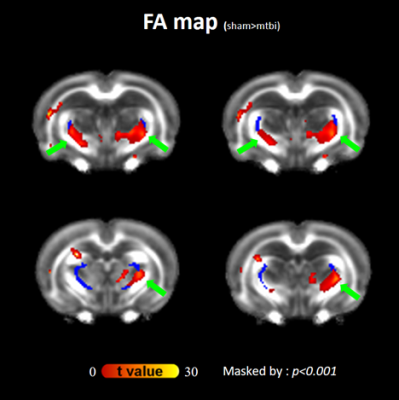
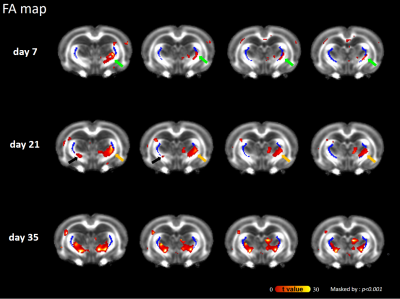
Figure 2. Evolution of injury in the statistical parametric maps At day 7, FA changes were observed only in unilateral TRN (green arrows) At day 21, FA changes with a larger extent were observed (yellow arrows) and some clusters of FA changes were noted on the contralateral side near TRN (black arrows). Finally, FA changes were seen in bilateral TRN at day 35.