1215
Α-ATP suppression in 31P MR spectroscopy by homonuclear BIRD filter: An approach to quantify NAD+ and NADH at 3T in vivo1Department of Radiology & Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 2Department of Nutrition & Movement Sciences, Maastricht University, Maastricht, Netherlands, 3Department of Radiology & Biomedical Imaging, Yale School of Medicine, New Haven, CT, United States
Synopsis
NAD+ and NADH play important roles in metabolism and metabolic health. Therefore, a non-invasive 31P MRS for the quantification of those metabolites on clinical 3T scanners was developed. This sequence suppresses α-ATP resonances based on homonuclear Bilinear Rotation Decoupling (BIRD). With reduced α-ATP intensity, neighbouring NAD+/H resonances appear as a separate peak. Results in eight young, lean and healthy volunteers show that the sequence succeeded in considerable α-ATP suppression. However, a clear separation of NADH and NAD+ resonances remains challenging due to remaining α-ATP signal.
Introduction
NAD+ and NADH play important roles as electron acceptor and donor respectively and high concentrations of NAD+ and a high ratio of NAD+/NADH are both strongly associated with metabolic health [1]. This makes the non-invasive quantification of these metabolites very interesting for metabolic research. On 3T clinical scanners however, there is severe overlap between α-ATP and NAD+/H resonances in 31P spectra [2]. Therefore, spectral editing of α-ATP is a promising way to visualize and quantify the underlying NAD+/H resonances. We used the principle of the Bilinear Rotation Decoupling (BIRD) filter [3] to suppress homonuclear coupled spins, such as α-ATP, based on their J-coupling constant. This new sequence was validated in vivo by measuring the NAD+/H levels in the lower legs of eight volunteers in basal state and during ischemia, as it is well known that ischemia increases NADH and decreases NAD+ concentrations [4].Methods
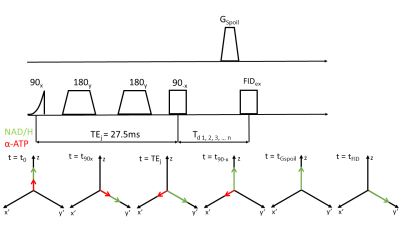
Eight young and healthy volunteers were positioned in the scanner (Achieva 3T, Philips Health Care, Best, the Netherlands) with an inflatable cuff around their upper leg. A 10cm single tuned 31P surface coil (Philips Health Care, Best, the Netherlands) was used to acquire FIDs in the lower leg in rest (deflated cuff) with NSA=32, points=2048, BW=3000Hz and TR=4000ms. Subsequently, homonuclear BIRD (HB) filtered spectra were acquired in rest and during eight minutes of ischemia created by inflating the cuff to a pressure of 50mmHG above systolic blood pressure. At TE=1/2J, α-ATP spins acquire a phase of 90⁰ with respect to uncoupled spins at in spin echo sequences [5] and we can see the spins as a rotated coordinate system where the z-axis is unchanged, uncoupled spins are create the axis x’ and coupled spins create the axis y’. Flipping spins about the y’-axis by -90⁰ results in uncoupled spins ending up aligned with the z-axis, whereas α-ATP spins remain in the x’-y’ plane and are dephased by a spoiler-gradient. A subsequent excitation only affects spins, which are aligned with z. Zero quantum coherences (ZQC) are removed by co-adding signals acquired with a variable delay Td between HB filter and FID excitation [6]. A depiction of the sequence is displayed in Figure 1. Scan parameters were as follows: NSA=128, points=2048, BW=3000Hz, TR=4000ms, TEj=27.5ms, Td=n/(-fβATP-fαATP) with n=1…32. In order to judge whether the HB filter is really necessary, we also aimed to determine NAD+/H levels from a simple FID. Spectra were analysed with the help of an in-house developed MATLAB script (MATLAB 2018b, The MathWorks,Inc.). The program first fitted resting state spectra and used the resulting fit parameters as starting values for fitting spectra acquired during ischemia. A Monte-Carlo simulation was performed for all data sets to determine Cramer-Rao lower bounds. Reproducibility of the HB filter sequence was tested by performing repeated in vivo measures (N=2) and comparison of their respective fitting results.Results
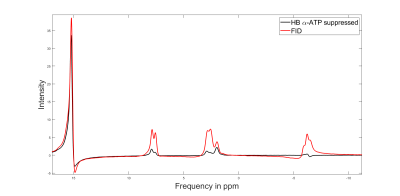
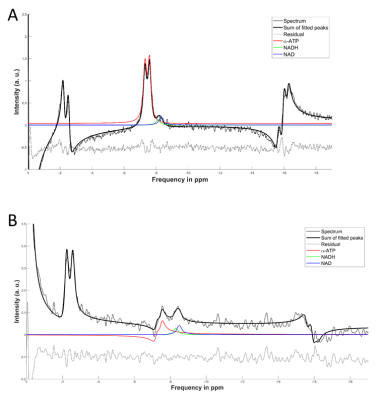
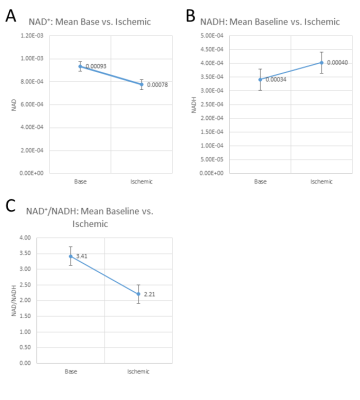
Figure 2 shows a comparison between FID and HB filtered spectra acquired from an ATP+NAD+ phantom. The HB filter thereby succeeded in suppressing α-ATP by 85%. Signal loss of NAD+/H between FID and HB filter spectra was ~30%. Reproducibility tests showed a coefficient of variation of 6.4% for NAD+ values, 10.8% for the sum of NAD+ and NADH, 36.9% for NADH. Ratios of NAD+/NADH varied by 62.8%. Results from Monte-Carlo simulations show an average relative Cramer-Rao lower bound of 25.3% for NADH and 11.1% for NAD+. Figure 4 shows results of resting state vs. ischemic spectra. NAD+ decreased significantly in ischemia (p<0.05), NAD+/NADH tended to decrease, while the increase in NADH was not significant (p=0.21). When quantified in a simple FID, the NAD+ and NADH signal intensities varied over a wide range with a prominent remaining residual in NAD+/H peak fits of the FID (Figure 3A), which is minimized in the edited spectrum. In addition, a clear separation of α-ATP is visible in the edited spectra compared to an FID (Figure 3B).Discussion
The homonuclear BIRD filter successfully suppresses a-ATP and uncovers NAD+/H resonances. This novel approach allows the observation of expected changes in NAD+ concentrations upon ischemia. However, the separation of NADH and NAD+ remains challenging and requires a fitting procedure with strong prior knowledge. As the NADH concentrations are generally lower than the NAD+ concentrations, the relative error was higher for the determination of NADH than for NAD+. From the results of the reproducibility, we can see that while reproducibility of NAD+ and the sum of NADH+NAD+ is good, the NADH quantification remains challenging. The variability in NADH also explains the variability of NAD+/NADH ratios. Residual α-ATP may result from suboptimal TEj, as the assumption of JαATP=18Hz [7] was probably not exactly correct as actual in vivo J-coupling constants of α-ATP appear to lie between 12 and 15Hz. To improve the performance of the HB filter further, an adjustment of TEj to match the in vivo J-coupling constants could be tested.Conclusion
The HB filter is a feasible approach for the detection of NAD+ and for the sum of NADH and NAD+. Further improvements such as optimizing TEj to reach a better α-ATP suppression might improve the accuracy of NAD+/NADH ratio quantification.Acknowledgements
No acknowledgement found.References
[1] L. R. Stein and S.-i. Imai, “The dynamic regulation of NAD metabolism in mitochondria,” Trends in Endocrinology & Metabolism, vol. 23, no. 9, pp. 420–428, 2012.
[2] M. Lu, X.-H. Zhu, and W. Chen, “In vivo 31P MRS assessment of intracellular NAD metabolites and NAD+/NADH redox state in human brain at 4 T,” NMR in Biomedicine, vol. 29, no. 7, pp. 1010–1017, 2016.
[3] J. Garbow, D. Weitekamp, and A. Pines, “Bilinear rotation decoupling of homonuclear scalar interactions,” Chemical Physics Letters, vol. 93, no. 5, pp. 504–509, 1982.
[4] K. Sahlin, “NADH and NADPH in human skeletal muscle at rest and during ischaemia,” Clinical Physiology, vol. 3, no. 5, pp. 477–485, 1983.
[5] P. C. van Zijl, C. T. Moonen, and M. von Kienlin, “Homonuclear j refocusing in echo spectroscopy,” Journal of Magnetic Resonance (1969), vol. 89, no. 1, pp. 28–40, 1990.
[6] M. Rance, G. Bodenhausen, G. Wagner, K. Wüthrich, and R. Ernst, “A systematic approach to the suppression of J cross peaks in 2D exchange and 2D NOE spectroscopy,” Journal of Magnetic Resonance (1969), vol. 62, no. 3, pp. 497–510, 1985.
[7] R. A. De Graaf, In vivo NMR spectroscopy: principles and techniques. John Wiley & Sons, 2013.
Figures