1213
Tracking cancer cells in the mouse brain with magnetic resonance imaging (MRI) and magnetic particle imaging (MPI)1University of Western Ontario, London, ON, Canada, 2Robarts Research Institute, London, ON, Canada
Synopsis
Magnetic particle imaging (MPI) is an emerging modality that may overcome challenges associated with quantification of iron-based MRI cell tracking. The magnetic properties of an SPION tracer heavily influences the sensitivity and resolution in MPI. Synomag-D™ has been explored as a sensitive tracer for MPI. Currently, no studies exist using this tracer to track cancer cells. Here, we utilize Synomag-D with MPI to detect and quantify iron-labeled breast cancer cells in the mouse brain and compare to MRI. Employing MPI for experimental cancer cell tracking will allow for quantification of the arrest, clearance, and retention of cancer cells in vivo.
Introduction
Magnetic particle imaging (MPI) is an emerging modality that sensitively and specifically detects the magnetization of superparamagnetic iron-oxide nanoparticles (SPIONs). Our lab has developed SPION based cellular magnetic resonance imaging (MRI) technology for many years to determine the fate of metastatic breast cancer cells to the brain.1–5 MRI cell tracking with SPIONs has some limitations. First, quantification of iron-induced signal loss detected with MRI is challenging. Second, other regions of signal void in MR images (ie. bone, air, blood) lead to low specificity. MPI cell tracking has the potential to overcome these challenges. The MPI signal appears as a hot spot with no background, providing high specificity. The main limitation of MPI, compared to MRI, is resolution. The MPI signal is also proportional to iron content and directly quantifiable, providing the ability to measure iron mass and estimate cell number. Sensitivity and resolution in MPI depend heavily on the magnetic properties of the SPION tracer. Synomag-D™, a nanoflower particle that has been shown to be superior to ferucarbotran, a commonly used MPI tracer, has been explored as a sensitive tracer for MPI. Currently, no studies exist that use this tracer to track cancer cells. Our aim was to utilize Synomag-D for quantitative MPI of iron-labeled breast cancer cells disseminated in the mouse brain and to compare to previous work where we used breast cancer cells labeled with micron sized iron particles (MPIO) and imaged by MRI.Methods
Human brain metastatic breast cancer cells (231BR) were labeled with Synomag-D, a dextran coated nanoflower iron-oxide particle with a core diameter of 30 nm (MicroMod GmbH). Labeling efficiency was determined with a Perls' Prussian Blue (PPB) stain to visualize intracellular iron. The number of positively stained cells in 3 random 40x magnification fields were counted. NOD/SCID/ILIIrg−/− (NSG) mice (n=6) were injected with 2.5x105 cells intracardially (IC) using ultrasound guidance. MPI was performed on a MOMENTUM scanner (Magnetic Insight Inc., Almeda, CA, USA) on Day 0 (n=3) and Day 7 (n=6). Images were collected using the 3D high sensitivity isotropic (multichannel) scan mode using a 5.7 T/m gradient, 21 projections, and a FOV 6x6x4 cm, for a scan time ~13 mins per mouse brain. MPI data was compared to MRI obtained in a prior study using mice injected IC with 2.5x105 MPIO-labeled 231BR cells.Results
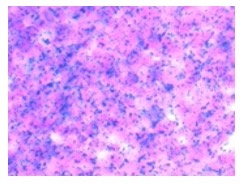
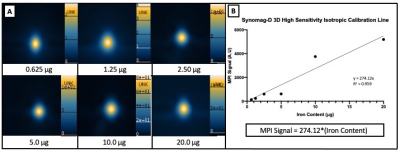
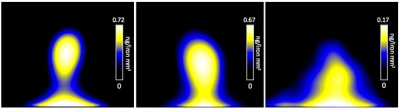
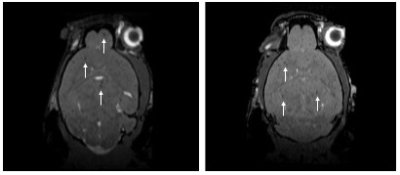
231BR cells were successfully labeled with Synomag-D with an efficiency of 98.0±0.40% (Figure 1). Cell viability was unaffected, as trypan blue exclusion assay revealed cells were 98% viable post-labeling. A calibration line was generated from samples of Synomag-D (Figure 2A) to determine iron content for a given MPI signal based on the 3D, high sensitivity, isotropic parameters. We determined there was a strong linear relationship between iron content and MPI signal (arbitrary units, A.U.) for Synomag-D (R2=0.96, p<0.0001) (Figure 2B). The equation of the line was: MPI Signal=274.12*(Iron Content). Using this relationship, iron content could be determined for a given MPI signal. Figure 3 shows representative in vivo MPI. On the day of the injection, MPI signal was detected in the brain in 3/6 mice. The mean iron content measured for these brains was 0.08 ug. Strong MPI signal was also visible in the lung/liver region since cells injected IC are distributed throughout the body. We have shown that such strong MPI signal impedes detection and quantification in nearby regions with low signal.6,7 Day 7 MPI revealed that lung/liver signal had diminished over time resulting in the ability to distinguish MPI signal in the brains of 5/6 mice at this time point. MPI signal in the brain on day 7 was lower, with mean iron content measuring 0.012 ug. This is expected, as a large proportion of arrested cancer cells are known to die and clear by day 7 in this model.1,8 In day 0 MRI, MPIO-labeled cells appear as discrete signal voids throughout the brain (Figure 4). Quantification of these images is challenging; voids can be counted, or the total signal void volume, or percentage of black pixels in the brain, can be calculated. However, this does not allow for the determination of cell number.Discussion
This is the first study to demonstrate that brain metastatic breast cancer cells can be labeled with Synomag-D, and subsequently, can be detected in vivo in the mouse brain with MPI. We calculated the iron content in each mouse brain using a calibration line which is used to relate MPI signal to known iron content in Synomag-D samples. This data indicated that we were detecting on average 16,000 cells in the mouse brain with a cell labeling of ~5 pg of iron/cell.Conclusion
Here, we utilize Synomag-D with MPI to sensitively detect iron-labeled breast cancer cells in the mouse brain and quantify cell number. Employing MPI for experimental cancer cell tracking will allow for the detection and quantification of the arrest, clearance, and retention of cancer cells in vivo.Acknowledgements
This project is supported by the Breast Cancer Society of Canada (BCSC) and the Translational Breast Cancer Research Unit (TBCRU), the Western Graduate Research Scholarship (WGRS), and the Ontario Graduate Scholarship (OGS).References
1. Parkins KM, Hamilton AM, Makela AV, Chen Y, Foster PJ, Ronald JA. A multimodality imaging model to track viable breast cancer cells from single arrest to metastasis in the mouse brain. Sci Rep. 2016;6(1):1–9.
2. Heyn C, Ronald JA, Mackenzie LT, et al. In vivo magnetic resonance imaging of single cells in mouse brain with optical validation. Magn Reson Med. 2006;55(1):23-29. doi:10.1002/mrm.20747
3. Heyn C, Ronald JA, Ramadan SS, et al. In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn Reson Med Off J Int Soc Magn Reson Med. 2006;56(5):1001–1010.
4. Knier NN, Hamilton AM, Foster PJ. Comparing the fate of brain metastatic breast cancer cells in different immune compromised mice with cellular magnetic resonance imaging. Clin Exp Metastasis. 2020;37(4):465-475. doi:10.1007/s10585-020-10044-0
5. Murrell DH, Zarghami N, Jensen MD, et al. MRI surveillance of cancer cell fate in a brain metastasis model after early radiotherapy. Magn Reson Med. 2017;78(4):1506–1512.
6. Makela AV, Gaudet JM, Schott MA, Sehl OC, Contag CH, Foster PJ. Magnetic Particle Imaging of Macrophages Associated with Cancer: Filling the Voids Left by Iron-Based Magnetic Resonance Imaging. Mol Imaging Biol. 2020;22(4):958-968. doi:10.1007/s11307-020-01473-0
7. Sehl OC, Gevaert JJ, Melo KP, Knier NN, Foster PJ. A Perspective on Cell Tracking with Magnetic Particle Imaging. other; 2020. doi:10.20944/preprints202009.0720.v1
8. Heyn C, Ronald JA, Ramadan SS, et al. In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn Reson Med Off J Int Soc Magn Reson Med. 2006;56(5):1001–1010.
Figures