1207
Radial tiny golden angle MRI combined with Cardiac and Respiratory Self-Gating in the Small Animal model – one acquisition, many possibilities1Internal Medicine II, Ulm University Medical Center, Ulm, Germany, 2Core Facility Small Animal Imaging (CF-SANI), Ulm University, Ulm, Germany
Synopsis
Radial tiny golden angle MRI is a versatile tool for different applications in the small animal model. Changes on short time-scales can be visualized by sliding-window (and Compressed Sensing) reconstructions, while respiratory and cardiac self-gating enable high-resolution images for cyclic motion patterns – all from the same acquisition.
Introduction/Purpose:
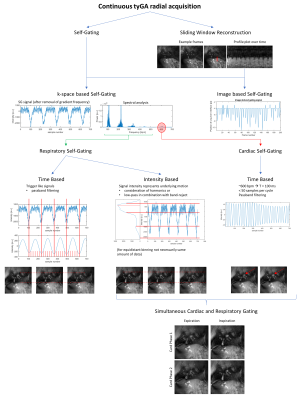
Compensation and assessment of motion is of paramount importance in small animal imaging1. Imaging techniques enabling high-quality motion-resolved imaging, while still being robust in case of residual motion appear highly demanded. While there exist a number of techniques to synchronize the acquisition with the ECG or respiration2, related artifacts arising from e.g. perturbation of the steady state or non-constant TR often limit the final image quality. Retrospective self-gating3,4,5 combining continuous data acquisition with subsequent reordering of the data into different motion bins has been introduced for Cartesian and conventional radial scanning. Even though solving some of the aforementioned limitations they are prone to artifacts due to nonuniform k-space coverage especially for short scan durations (Figure 1).Tiny golden angles6,7 were introduced as a surrogate for the golden angle, yielding nearly uniform azimuthal profile distribution for an almost arbitrary number of profiles while reducing eddy current related artifacts. tyGA echo acquisitions have enabled real-time functional imaging of mouse hearts with a temporal resolution as low as 16.8ms8. Furthermore, the tyGA approach has been combined with self-gating in human application, e.g. for fetal cardiac MRI9 or whole heart imaging10. In this contribution, we like to show the flexibility of the tyGA technique, which enables reconstruction of sliding-window real-time images with free choice of the temporal resolution (e.g. needed for cardiac or lung perfusion assessment), and high-resolution cardiac and/or respiratory self-gated images (e.g. required for cardiac or lung function assessment) from a single continuously acquired data set.
Methods:
All imaging experiments were performed on an 11.7Tesla dedicated small animal MRI system (BioSpec 117/16, Bruker Biospin, Ettlingen, Germany), equipped with a four-element thorax coil (RAPID Biomedical, Rimpar, Germany), sequence parameters are provided in Table 1. Reconstruction was performed with an in-house developed MATLAB framework. Required self-gating signals were extracted from the center of k-space (DC) and subsequently filtered according to the envisaged application (Figure 2). For cardiac self-gating, the gating signal was by applying a bandpass filter around the cardiac frequencies as seen in Fourier analysis. Binning was performed on the time axis. For respiratory self-gating, the characteristic of the respiratory process was conserved by including the harmonics of the fundamental respiratory frequencies during filtering. The respiratory plateau used for binning was derived from histogram analysis.Results:
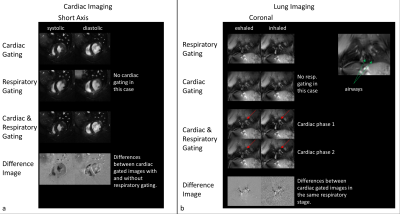
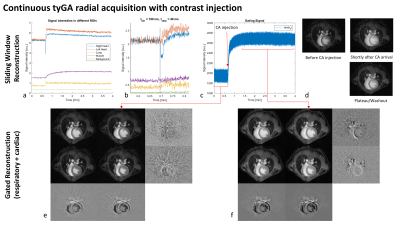
Examples of the resulting image quality from respiratory and/or cardiac self-gating from continuously acquired tyGA data are shown in Figs.3 and 4. The images clearly reveal the possibility of deriving flexible gating information from the continuous data stream thus enabling gated reconstruction for cardiac and respiratory motion (Fig.3). Please note the extremely low motion artifact level in cases where only a single motion is considered. Due to the properties of the tyGA acquisition, residual motion mainly yields image blur instead of distinct motion artifacts. The same technique can also be applied for real-time imaging (Fig.4) e.g. for the visualization of contrast agent (CA) dynamics after systemic CA injection. Due to the unique homogenous k-space coverage of tyGA, the continuous data stream acquired during the injection can be used to reconstruct the data at a flexible temporal resolution (trade-off temporal fidelity vs. SNR). However, the same data can also be used for high-quality self-gated imaging of the lung/heart e.g. to compare contrast properties prior and after CA injections. After administration of CA to the tail vein, signal changes can first be seen in the right ventricle and shortly after in the lung and the left heart. The change in muscle intensity is slower, but remains constant for the duration of the scan, while the heart and to a lesser extent the lung show a declining signal intensity, corresponding to CA tissue uptake/blood washout. The background noise level is not influenced.Discussion:
In the animal, respiratory motion does not influence cardiac gated images as strongly as in human application. This is due to the respiratory pattern, which consists of a long expiration plateau period and a short ‘gasp’ for air. However, in direct comparison, cardiac imaging was shown to benefit from the consideration of respiratory motion. Differences are especially prominent in the vessels, although this might be considered a cosmetic improvement. However, differences in the heart itself, possibly caused by through-plane motion during respiration, may lead to differences in volumetric measurements and could be further investigated in the future. Lung imaging benefits greatly from the consideration of cardiac motion. The advantage in axial scans is obvious as the contracting heart makes up a large part of the torso. While the heart itself might not be present in every coronal slice of a scan, the motion of the large vessels can be derived from the self-gating signal. This does not only enable the detection of changes over the heartbeat (perfusion) but also improves image quality for morphologic imagingConclusion:
The tiny golden angle trajectory can be used as a basis for various reconstruction methods. We showed in this work, that besides real-time sliding-window reconstructions, simultaneous cardiac and respiratory gating, tailored to the desired application, is possible in small animal applications. This enables the extraction of a variety of information from a single acquisition and could greatly simplify the small animal imaging process.Acknowledgements
The authors thank the Ulm University Centre for Translational Imaging MoMAN for its support.References
(1) Li et al., Front Phys 2020, 10
(2) Runge et. al., Radiology 1984, 151
(3) Hoerr et. al., J Cardiovasc Magn Reson 2013, 15 (59)
(4) Larson et. al., Magn Reson Med 2004, 51 (1)
(5) Tibiletti et. al., Magn Reson Med 2016, 75 (6)
(6) Wundrak et. al., IEEE TMI 2014, 34 (6)
(7) Wundrak et. al., Magn Reson Med 2016, 75 (6)
(8) Li et. al., NMR Biomed 2020, 33 (7)
(9) Haris et. al., J Magn Reson Imaging 2017, 46 (1)
(10) Zhang et. al., Magn Reson Med 2018, 81 (5)
Figures