1205
In vivo characterization of effect of microglia regeneration after high altitude exposure by quantitative MRI in mice1Radiology and Radiological Sciences, Uniformed Services University, Bethesda, MD, United States, 2Center for Neuroscience and Regenerative Medicine, Henry M. Jackson Foundation, Bethesda, MD, United States, 3Department of Pharmacology & Molecular Therapeutics, Uniformed Services University, Bethesda, MD, United States, 4Department of Pathology, Uniformed Services University, Bethesda, MD, United States, 5Department of Anatomy, Physiology and Genetics, Neuroscience Program, Uniformed Services University, Bethesda, MD, United States
Synopsis
Prolonged stays in high-altitude (HA) environments produce pro- and maladaptive physiological and pathological changes. Microglia depletion has been utilized in recovery from a spectrum of neurodegenerative diseases abnormalities. Advanced neuroimaging techniques demonstrate great promise to detect subtle changes in brain activity and morphology related to impairments induced by HA. In this work, we applied advanced MRI and modern behavioral tests to investigate neuropathological consequences of hypobaric hypoxia and the use of microglial regeneration as a novel approach to treat the maladaptive effect of HA.
Introduction
Exposure to hypobaric hypoxia has been shown to cause changes in brain physiology and function, such as headaches, sleep disorders, and other sequela.1-3 We have shown that chronic exposure to HA results in cognitive deficits, changes in white matter integrity and microglial activation.4 Microglia are the principal immune cells of the central nervous system (CNS) and are versatile players in both inflammatory and physiological processes.5 Depletion and regeneration of microglia has been used in recovery from a spectrum of neuroinflammatory insults and improved learning and memory in an aged Alzheimer’s disease mouse model,6 as well as age-related changes in neuronal gene expression.7Magnetic resonance imaging (MRI) provides non-invasive detection of white matter reorganization and may detect subtle changes in brain activity and morphology related to impairments induced by HA, including changes in vasculature and microglia development.4 The objective of this study was to use advanced MRI and behavioral examinations to investigate neuropathological consequences of hypobaric hypoxia and the use microglial regeneration as a novel approach to treat the maladaptive effect of HA.
Methods
Forty male mice approximately 7 weeks of age were ordered from Jackson Laboratories (C57BL/6J). Mice were split into four groups: sea level (SL) and normal chow, 2) SL and a microglial depletion (PLX5622) diet, 3) HA for 3 weeks at 5000 meters and normal chow, and 4) HA and PLX diet. CO2 and O2 levels, temperature, relative humidity, and barometric pressure were monitored and recorded within the hypobaric chamber.Memory impairment was assayed using a fear conditioning paradigm, to assess contextual and cued fear memory. Spatial memory in the Y maze was also tested in these animals. These behavioral tests were performed at 2, 4 and 8 weeks after microglia repopulation.
MRI experiments were conducted using a 7 T Bruker Biospec 70/20 (Bruker Biospin, Billerica, MA) equipped with high-performance actively shielded gradients (660 mT/m) with integrated shim coils. A birdcage RF transmit coil in combination with actively decoupled 4-channel RF receive array coil was used. MRI experiments were conducted following the final behavioral testing, 8 weeks after microglia repopulation.
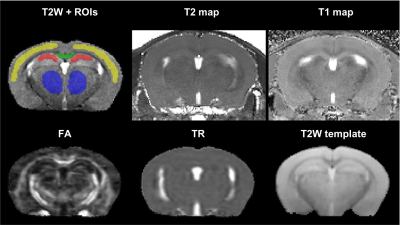
Acquired MRI data included: fast spin-echo T2 (100×100×600 microns, TR/TE= 4000/ (10/30/50/70/90/110) ms, RARE factor= 2, 4 averages); MP2RAGE (100×100×600 microns, TR/TE/TI1/TI2= 6400/3/270/2200 ms); DTI (2D EPI, repeat set with phase encode direction reversed, 200 microns isotropic, TR/TE=4000/32 ms, 4 B0 volumes, 30 volumes with b=1000s/mm2, 8 averages). T2-maps were computed in Matlab R2020a (Mathworks, MA, USA), and T1-maps were computed using IDL (Harris Geospatial Solutions, Inc.). The DTI data was processed using TORTOISE v3.2.08 with DR-BUDDI9 to correct for EPI-induced distortions; fractional anisotropy (FA) and trace (TR) maps were computed. A brain mask was created from the 30 ms T2 and T2-map automatically using DEMON.10 The 30 ms T2 was resampled to isotropic resolution using SMORE,11 which was then nonlinearly registered to a template using the SyN algorithm in ANTs.12 Four regions of interest were manually segmented covering the central portion of the brain (Figure 1): mid portion of corpus callosum, hippocampus, neocortex, and thalamus.
Results and Discussion
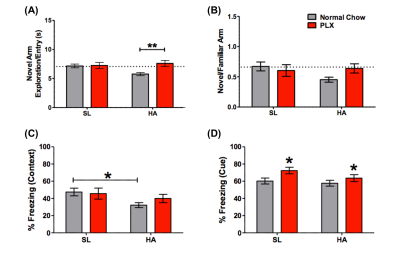
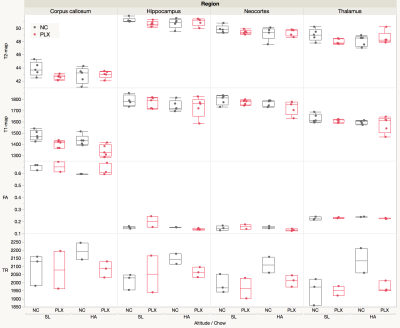
HA exposure and PLX treatment altered spatial memory in the Y maze assay (Altitude by Treatment interaction on novel arm exploration time per entry; F(1, 54) = 4.178, p = 0.0458, Figure 2A). PLX treatment reversed the decrease in novel arm exploration in HA exposed mice. There was a trend towards a significant interaction for the ratio of overall time spent in the novel/familiar arms (Figure 2B). Deficits in contextual fear memory were apparent following HA exposure (main effect of altitude, (F(1, 39) = 4.343, p = 0.0438, Figure 2C), but not significantly altered by PLX treatment. In contrast, cued fear memory was enhanced by PLX treatment, but unaltered by altitude (main effect of PLX treatment; F(1, 39) = 5.361, p = 0.0259, Figure 4D).The representative T2W, T2, T1, FA and TR maps images with calculated using ROIs in four selected regions are shown in Figure 1 and 3 respectively. The T1 values indicate an interesting trend in the corpus callosum, which may reflect improving of white matter integrity due to PLX treatment regardless of HA exposure. The trace values were elevated in all regions for not treated (normal chow) HA group, which could correlate with our previous results (not shown here) of the increased cerebral blood flow (CBF) in HA conditioned mice. There is an observable decrease of trace of the diffusion tensor (TR) values to the baseline level after PLX treatment of HA exposed mice suggesting reduction in maladaptive effect of HA by PLX treatment.
Conclusions
The behavioral result suggests that PLX treatment may restore spatial memory and enhance cued fear memory in mice exposed to HA. MRI analyses suggest that PLX treatment could counteract the structural changes and behavioral effects of chronic HA exposure.Acknowledgements
This work was funded by the U.S. DOD in the Center for Neuroscience and Regenerative Medicine and U.S. Department of Defense Program Project 308430 USUHS. We want to thank Frank Ye (NIH NIMH) for providing MP2RAGE pulse sequence for Bruker’s Paravision 6.1. We want to thank Plexxikon Inc. for providing PLX5622.
Disclaimer: The views expressed in this scientific presentation are those of the authors and do not necessarily reflect the official policy or position of the U.S. government, the Department of Defense or the Uniformed Services University.
References
- Boroujerdi A and Milner R. Defining the critical hypoxic threshold that promotes vascular remodeling in the brain. Exp. Neurol. 263, 132–140 (2015).
- LaManna, JC, Chavez JC, and Pichiule P. Structural and functional adaptation to hypoxia in the rat brain. J. Exp. Biol. 207, 3163–3169 (2004).
- Xu K, Puchowic, MA, and LaManna JC. Renormalization of regional brain blood flow during prolonged mild hypoxic exposure in rats. Brain Res. 1027, 188–191 (2004).
- Cramer NP, Korotcov A, Bosomtwi A. et al. Neuronal and vascular deficits following chronic adaptation to high altitude. Exp Neurol. 311:293-304 (2019).
- Han J, Harris RA, Zhang X-M. An updated assessment of microglia depletion: current concepts and future directions. Molecular Brain. 2017;10(1):25.
- Dagher NN, Najafi AR, Neely Kayala KM et al. Colony-stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3xTg-AD mice. J Neuroinflammation. 2015 Aug 1;12:139.
- Elmore MRP, Hohsfield LA, Kramár EA et al. Replacement of microglia in the aged brain reverses cognitive, synaptic, and neuronal deficits in mice. Aging Cell. 2018 Dec;17(6):e12832
- Pierpaoli C, Walker L, Irfanoglu MO et al. 2010, TORTOISE: an integrated software package for processing of diffusion MRI data, ISMRM 18th annual meeting, Stockholm, Sweden, abstract #1597
- Irfanoglu MO, Modi P, Nayak A et al. DR-BUDDI (Diffeomorphic Registration for Blip-Up blip-Down Diffusion Imaging) method for correcting echo planar imaging distortions, Neuroimage. 2015 Feb 1;106:284-99. doi: 10.1016/j.neuroimage.2014.11.042.
- Roy S, Knutsen AK, Korotcov A et al. A deep learning framework for brain extraction in humans and animals with traumatic brain injury. 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018). 2018; :687-691. doi: 10.1109/ISBI.2018.8363667.
- Zhao C, Dewey BE, Pham DL et al. SMORE: A Self-supervised Anti-aliasing and Super-resolution Algorithm for MRI Using Deep Learning. November 2020IEEE Transactions on Medical Imaging PP(99):1-1. DOI: 10.1109/TMI.2020.3037187
- Avants BB, Epstein CL, Grossman M, and Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain.Med Image Anal 2008 Feb;12(1):26-41.
Figures