1203
Contrast enhancement of the normal infundibular recess using 3D FLAIR1Saitama Medical University Hospital, Saitama, Japan
Synopsis
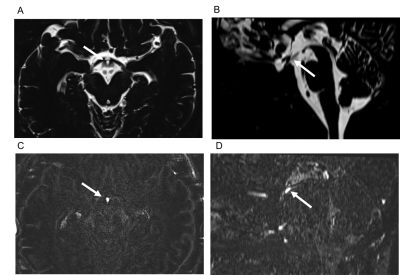
The infundibular recess (IR) is a cerebrospinal fluid (CSF) space in the third ventricle floor, and its function remains unclear. We retrospectively evaluated contrast enhancement of the normal IR using heavily T2-weighted 3D FLAIR. The enhancement of IR was the strongest on post-contrast images, followed by 4-h delayed post-contrast images. It was also stronger than that of other CSF spaces. This is the first study to report the enhancement of IR after an intravenous gadolinium injection. Evaluations of the enhancement of IR may clarify substance transport, such as gadolinium or hormones into the CSF.
Introduction
The infundibular recess (IR) is a cerebrospinal fluid (CSF) space in the third ventricle floor that is surrounded by the median eminence, one of the circumventricular organs (CVOs) characterized by permeable fenestrated capillaries lacking a blood-brain barrier (BBB). Therefore, substances may move freely between the blood and CVOs. Previous studies demonstrated that the median eminence was enhanced on T1-weighted or FLAIR imaging after the intravenous administration of gadolinium [1, 2]. However, enhancement of the normal IR has not yet been reported. In our clinical practice, IR was enhanced on heavily T2-weighted 3D FLAIR (HT2-FLAIR). HT2-FLAIR is a variant of the 3D FLAIR sequence with variable flip-angle refocusing pulses [3]. This sequence increases sensitivity to low concentrations of contrast material over that of conventional 3D FLAIR. A previous study showed enhancement of the anterior eye segment and various areas of the cranial subarachnoid space on HT2-FLAIR [4]. Although IR has been linked to the endocrine system [5, 6], its function remains unclear. Kanda et al. detected gadolinium deposits in the brain after intravenous administration [7]. Although the exact mechanism remains unclear, several hypotheses have been proposed, one of which is the glymphatic system. The glymphatic system is a functional waste clearance pathway for the central nervous system [8]. This system drives CSF into the interstitial space of the brain along the perivascular spaces surrounding arteries and out along those surrounding veins. Therefore, evaluations of IR enhancement may clarify gadolinium transport into CSF and its deposition in the brain. The present study aimed to evaluate contrast enhancement of the normal IR using HT2-FLAIR.Methods
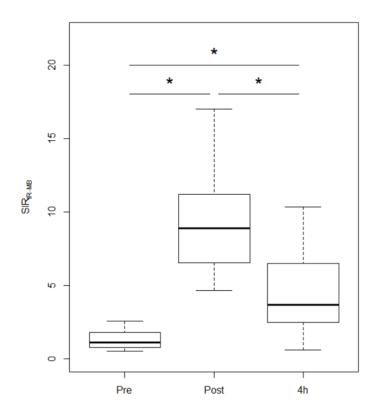
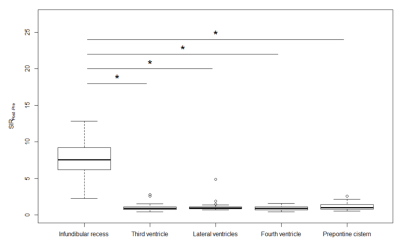
Twenty-six patients (mean 54 years, male/female 11/15) were retrospectively recruited. We subjectively compared the overall enhancement of IR on post-contrast, 4-h delayed HT2-FLAIR, and pre-contrast images. We also objectively conducted chronological and spatial comparisons by measuring the signal intensity (SI) ratio (SIR). Chronological comparisons were conducted by comparing SIR IR-MB (SI of IR/SI of the mid-brain) using the Friedman test followed by the Holm correction. Spatial comparisons were performed by comparing the SIR Post- Pre (SI on post-contrast HT2-FLAIR/SI on pre-contrast HT2-FLAIR) of IR with that of other CSF spaces, including the superior part of the third ventricle, lateral ventricles, fourth ventricle, and prepontine cistern, using the Kruskal-Wallis test followed by the Steel test. p values < 0.05 were considered to be significant.Results
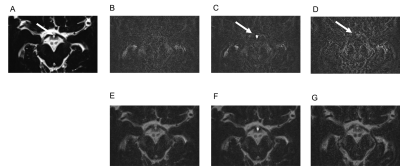
In a subjective analysis, the enhancement of IR on post-contrast and 4-h delayed post-contrast HT2-FLAIR was observed in all cases, and enhancement was weaker on 4-h delayed post-contrast HT2-FLAIR than on post-contrast HT2-FLAIR (Figure 1 and 2). In an objective analysis, SIR IR-MB was the highest on post-contrast images, followed by 4-h delayed post-contrast images (Figure 3). SIR Post-Pre was significantly higher in IR than in other CSF spaces (Figure 4).Discussion
It is unknown why IR showed contrast enhancement. One possible explanation is the leakage of contrast material into CSF from the median eminence, a CVO. Although CVOs are functionally and morphologically diverse, they have several common features: 1) fenestrated capillaries lacking BBB, 2) specialized neuroglial cells (tanycytes and pituicytes), and 3) an interface between the brain, blood, and CSF. These organs play a critical role as a transducer of information between the brain, blood, and CSF. The median eminence is characterized by permeable fenestrated capillaries lacking BBB. Therefore, substances may move freely between the blood and extracellular space within the median eminence. The dorsal wall of the median eminence bordering the floor of IR is lined by β2 tanycytes. Tight junctions between β2 tanycytes at the ventricular pole restrict the passage of substances between CSF and the extracellular space within the median eminence. However, previous studies reported the transport of substances from the median eminence to CSF in the third ventricle [5, 9, 10]. Caraty et al. demonstrated that gonadotropin-releasing hormone (GnRH) was not uniformly distributed throughout the third ventricle and was more concentrated in IR [5]. They hypothesized that the median eminence may be the major, if not only, source of GnRH entering CSF. Other studies revealed that peptide hormones, including leptin and ghrelin, were transported after peripheral administration from the blood to CSF via tanycytes in the median eminence [9, 10]. Therefore, tight junctions between β2 tanycytes may be leaky. Gadolinium deposition in the brain after intravenous administration was initially reported by Kanda et al. [7]. Recent findings suggest a role for the glymphatic system as one of the transport pathways of gadolinium into the brain parenchyma [11-16]. Furthermore, intravenously administered gadolinium was distributed to perivascular spaces [14] as well as CSF spaces around various tissues, including the peripheral part of the cranial nerves [4] and the cortical veins [16]. The perivascular space comprising the glymphatic system functions as a transport conduit of CSF and solutes in the brain. The constellation of these MR findings suggests the involvement of the glymphatic system in the transportation and deposition of gadolinium in the brain.Conclusion
The present results demonstrated that IR was enhanced on HT2-FLAIR. IR is a potential source of the leakage of intravenously administered gadolinium into CSF.Acknowledgements
We express our appreciation to all the technicians, nurses, and patients involved with the study.References
1. Horsburgh A, Massoud TF. The circumventricular organs of the brain: conspicuity on clinical 3T MRI and a review of functional anatomy. Surg Radiol Anat. 2013;35(4):343-9.
2. Tsutsumi S, Ono H, Yasumoto Y. The tuber cinereum as a circumventricular organ: an anatomical study using magnetic resonance imaging. Surg MethodsRadiol Anat. 2017;39(7):747-51.
3. Naganawa S, Yamazaki M, Kawai H, Bokura K, Sone M, Nakashima T. Visualization of endolymphatic hydrops in Ménière's disease with single-dose intravenous gadolinium-based contrast media using heavily T(2)-weighted 3D-FLAIR. Magn Reson Med Sci. 2010;9(4):237-42.
4. Naganawa S, Yamazaki M, Kawai H, Sone M, Nakashima T. Contrast enhancement of the anterior eye segment and subarachnoid space: detection in the normal state by heavily T2-weighted 3D FLAIR. Magn Reson Med Sci. 2011;10(3):193-9.
5. Caraty A, Skinner DC. Gonadotropin-releasing hormone in third ventricular cerebrospinal fluid: endogenous distribution and exogenous uptake. Endocrinology. 2008;149(10):5227-34.
6. Robinson AG, Zimmerman EA. Cerebrospinal fluid and ependymal neurophysin. J Clin Invest. 1973;52(5):1260-7.
7. Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270(3):834-41.
8. Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra11.
9. Balland E, Dam J, Langlet F, Caron E, Steculorum S, Messina A, et al. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab. 2014;19(2):293-301.
10. Collden G, Balland E, Parkash J, Caron E, Langlet F, Prevot V, et al. Neonatal overnutrition causes early alterations in the central response to peripheral ghrelin. Molecular metabolism. 2015;4(1):15-24.
11. Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123(3):1299-309.
12. Ringstad G, Vatnehol SAS, Eide PK. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain. 2017;140(10):2691-705.
13. Dyke JP, Xu HS, Verma A, Voss HU, Chazen JL. MRI characterization of early CNS transport kinetics post intrathecal gadolinium injection: Trends of subarachnoid and parenchymal distribution in healthy volunteers. Clin Imaging. 2020;68:1-6.
14. Naganawa S, Nakane T, Kawai H, Taoka T. Gd-based Contrast Enhancement of the Perivascular Spaces in the Basal Ganglia. Magn Reson Med Sci. 2017;16(1):61-5.
15. Naganawa S, Taoka T, Kawai H, Yamazaki M, Suzuki K. Appearance of the Organum Vasculosum of the Lamina Terminalis on Contrast-enhanced MR Imaging. Magn Reson Med Sci. 2018;17(2):132-7.
16. Ohashi T, Naganawa S, Ogawa E, Katagiri T, Kuno K. Signal Intensity of the Cerebrospinal Fluid after Intravenous Administration of Gadolinium-based Contrast Agents: Strong Contrast Enhancement around the Vein of Labbe. Magn Reson Med Sci. 2019;18(3):194-9.
Figures