1202
Is MRI Contrast Stable in High-Energy Radiation of MR-Linac?1Radiation Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, United States, 2Institute for Applied Cancer Science, University of Texas MD Anderson Cancer Center, Houston, TX, United States, 3Radiation Oncology, Mayo Clinic, Jacksonville, FL, United States, 4Radiation Physics, University of Texas MD Anderson Cancer Center, Houston, TX, United States, 5Radiation Oncology, University of Maryland, Baltimore, MD, United States
Synopsis
Gadolinium-based MRI contrast agents are deemed safe to use in diagnostic exams. Their use would bring value to radiation oncology applications such as daily replanning on the MR-Linac. However, their stability and safe use in the presence of high-energy radiation remains unknown. We analyzed the chemical composition stability of two common MRI contrast agents that were irradiated to various doses using mass spectrometry. We found no detectable amounts of degradation compounds or conformational alterations as a result of irradiation. This is the first step towards demonstrating the safe use of gadolinium-based MRI contrast agents in radiation oncology applications.
Introduction
Gadolinium (Gd)-based contrast is often used when acquiring MR images for radiation therapy planning for better target delineation1. In some situations, patients may still have residual MRI contrast agents in their tissue while being treated with high-energy radiation. This is especially true when MRI contrast agents are administered during adaptive treatment replanning for patients treated on MR-Linac systems2. In diagnostic exams, these contrast agents are considered safe and lead to adverse reactions in less than 1% of cases3,4. However, transmetallation of chelated Gd and free zinc in the blood can lead to the deposition of insoluble Gd-containing salts in tissue, which is believed to cause localized fibrotic changes and collagen deposition and can lead to nephrogenic systemic fibrosis5. Thus, chelation stability is the primary safety consideration for the use of Gd contrast agents, especially in patients with compromised renal function. The stability and potential toxicity of contrast agents during irradiation have become critical issues in patient care and safety in radiation oncology6. Several organizations, including ISMRM, have called for additional data-driven research investigating the safe use of Gd contrast agents, including during treatments such as chemotherapy and radiation therapy7. As a first step in addressing this gap in knowledge, the purpose of this study was to analyze the molecular stability of MRI contrast agents when exposed to high energy photons and the associated secondary electrons in a 1.5T MR-Linac system.Methods
Two common Gd-based MRI contrast agents (gadobutrol, gadobenate dimeglumine) were prepared to the same concentration that is injected during diagnostic imaging exams (8 mmol bolus for representative 80kg patient, which is evenly distributed in 5100mL blood volume). The diluted contrast agents were placed in vials and placed at isocenter of the 1.5T MR-Linac and irradiated with 7MV photons to doses of approximately 2, 8, 15, or 30Gy; two non-irradiated vials were used as baselines for comparison (controls). The samples were analyzed using liquid chromatography high resolution mass spectrometry (LC-HRMS) to detect degradation products or conformational alterations created by irradiation with high energy photons and associated secondary electrons. The average and standard deviation of the peak areas associated with the contrast agents across the irradiation conditions was calculated along with the coefficient of variation (CV) to measure the dispersion of the values. The correlation of peak area with dose was measured by calculating the Pearson correlation coefficient, and correlations that produced two-tailed p-values < 0.05 were deemed significant.Results
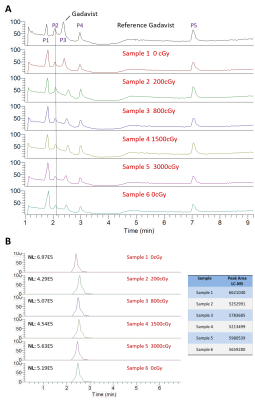
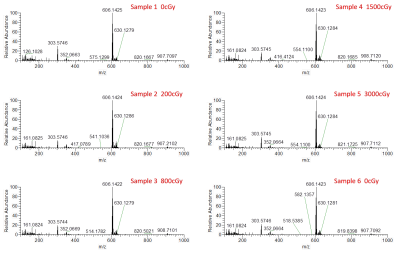
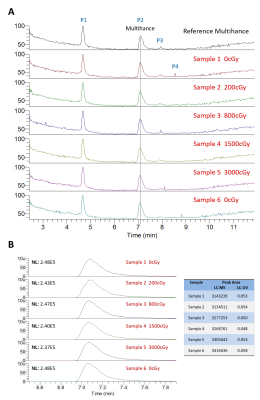
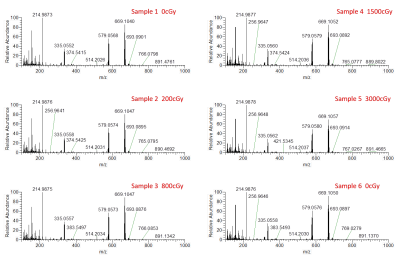
Five detectable peaks (P1-P5) were observed on each of the LC-HRMS chromatograms of the reference, unirradiated, and irradiated gadobutrol samples (Fig. 1). Qualitatively, the LC-HRMS profiles of the reference and unirradiated samples were identical to that of the irradiated samples. Average peak area for gadobutrol (P3) was 5751672 520986 (CV = 0.09), and there was no significant correlation between dose and peak area (R2 = 0.00, p = 0.90). The mass spectra of the ions in P3 of each individual gadobutrol sample indicated that none of these samples showed any detectable new ions following irradiation (Fig. 2). Similarly, the LC-HRMS profiles of the reference and unirradiated gadobenate dimeglumine samples were identical to that of the irradiated samples (Fig. 3). Average peak area for gadobenate dimeglumine (P2) was 3257574 106097 (CV = 0.03), and there was no correlation between dose and peak area (R2 = 0.11, p = 0.52). The ions in P2 of each individual gadobenate dimeglumine sample indicated that none of these samples showed any detectable new ions following irradiation (Fig. 4). Collectively, these results indicate that there is no molecular change in either contrast agent as a result of irradiation to clinical dose levels.Discussion
Qualitatively, there was no visible change in LC-HRMS spectra between the irradiated samples and unirradiated/reference samples for either contrast agent. The absence of new peaks in the irradiated samples suggests that no significant amounts of degradation compounds or conformational alterations were created as a result of the high-energy irradiation. This was also observed in the mass spectra of total ions and the mass spectra of individual peaks. Second, the quantification of the peak areas was uniform among all the samples, and there was no significant correlation of peak area with dose. The consistency between the peak areas and lack of negative correlation with dose suggests that the concentration of the contrast agent was not reduced due to potential conversion to new products. These conclusions are consistent with another study8 which found that the irradiation of MRI contrast agents led to a change in T1 relaxivity by < 1%. Our work builds from these findings by measuring direct chemical composition changes using mass spectrometry, which should theoretically be more sensitive.Conclusion
Mass spectrometry analysis indicated that MRI contrast agents were minimally affected by high-energy radiation in the presence of strong magnetic fields. The chemical composition stability of the irradiated contrast agents is promising for future use throughout the course of patient treatment. However, in vivo toxicity studies are needed to confirm that unexpected metabolites are not created in biological milieus.Acknowledgements
Supported in part by Cancer Center Support (Core) Grant P30 CA016672 from the National Cancer Institute, National Institutes of Health, to The University of Texas MD Anderson Cancer Center. We would also like to thank Christine Wogan for her assistance in editing the manuscript and for her insights.References
1. Griffin, B. R. et al. Improved Radiotherapy Treatment Planning for Spinal Cord Tumors Using Gadolinium Enhanced MR Imaging. Med. Dosim. 14, 5–8 (1989).
2. Sahin, B. et al. First 500 Fractions Delivered with a Magnetic Resonance-guided Radiotherapy System: Initial Experience. Cureus (2019) doi:10.7759/cureus.6457.
3. Shellock, F. G. & Kanal, E. Safety of magnetic resonance imaging contrast agents. J. Magn. Reson. Imaging 10, 477–484 (1999).
4. Jung, J.-W. et al. Immediate Hypersensitivity Reaction to Gadolinium-based MR Contrast Media. Radiology 264, 414–422 (2012).
5. Morcos, S. K. Extracellular gadolinium contrast agents: Differences in stability. Eur. J. Radiol. 66, 175–179 (2008).
6. Groot Koerkamp, M. L. et al. Optimizing MR-Guided Radiotherapy for Breast Cancer Patients. Front. Oncol. 10, (2020).
7. Gulani, V., Calamante, F., Shellock, F. G., Kanal, E. & Reeder, S. B. Gadolinium Deposition in the Brain: Summary of Known Science and Recommendations from the International Society for Magnetic Resonance in Medicine. Magn. Reson. Imaging 34, (2016).
8. Gultekin, D. H., Raidy, T. E. & Gore, J. C. Effects of radiation on the NMR relaxation effects of aqueous solutions of gadolinium contrast agents. Contrast Media Mol. Imaging 4, 33–36 (2009).
Figures