1197
PAA-g-(DTPA-Gadolinium): A Versatile Agent to Visualize the Effiencient Interstitial Stream at 7 Tesla1National Centre for Nanoscience and Technology, Beijing, China, 2University of Chinese Academy of Sciences, Beijing, China, 3Beijing University Of Chemical Technology, Beijing, China
Synopsis
Interstitium space and interstitial stream compartmentalize organs distributed in different regions and compose human body among parenchymal tissues. The increase in interests to investigate the behavior and function of interstitial stream, combined with new methodologies propose the demand in new agent to visualize the path itself. A macromolecule gadolinium-based agent PAA-Gd was introduced to outline the path while mass transportation was driven in Balb/c via interstitial stream and histology study was combined to perform in microscopic view as tracer. The novel agent can display its high efficiency to visualize the stream in radiology and pathology study as a versatile enhancer.
INTRODUCTION
The interstitial stream in tissues and among organs is a major fluid compartment in hierarchical porous media in human. A long-range transport in loose connective tissue (LCT) along veins was found and a tailed research on artificial fiber network was done to simulate the transportation speed described with Fick’s Law1,2. Our previous in vitro study has confirmed the LCT long-range transmission by using atomic force microscopy cantilever knock at the end of adventitia of blood vessel and characterized the super lubrication of LCT and came into the emphasis in soft matter researches2-4 and has been focused on biodistribution and transportation of nanoparticles and liquid metal, both confirmed directional transport which differed from that of blood transport over long distance5-7. However, the long distance transportation along the interstitial stream has not been captured in vivo. Also the agent to perform as transportation mass and contrast medium is demanded. To address these challenges, our study applied a macromolecular magnetic resonance (MR) imaging agent via intervaginal space injection (ISI) 5,7 to accomplish in vivo study and enhance the contrast of interstitial stream in situ during scanning on Balb/c mice. The injection gap positions at tarsal tunnel and consists of LCT which is connected into interstitial stream. The macromolecule agent chelated a long chain with Gd3+ and exhibited high longitudinal relaxitivity. Dynamic contrast enhanced (DCE) MRI 8 was carried out to illustrate feasibility of polyacrylicacid)-g-(DTPA- Gadolinium) (PAA-Gd) agent and histochemical study was further insighted to validate this approach at microscopic view.METHODS
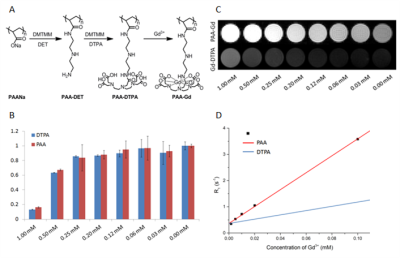
Polyacrylate-diethylenetriamine (PAA-DET) polymer was first synthesized and then pendant diethylenetriaminepentaacetic acid (DTPA) groups were added to form PAA-DTPA. GdCl3·6H2O was dissolved and dripped in the previous synthesized solution. 3k MWCO centrifugal devices were used to remove the free Gd3+ ions and convert the solvent to 1× PBS. Cytotoxicity assessed on HUVEC and was determined with both PAA-Gd and clinically accepted Gd-DTPA. Phantom test and in vivo imaging on Balb/c was carried out on a 7T scanner. The PAA-Gd agent was intervaginally injected with indwelling needle in the tarsal tunnel formed by medial malleolus of the tibia at posteromedial side of ankle via ISI method 5,6 while MR scanning. Dynamic contrast enhanced (DCE) images were acquired from pre-injection to approximately 30 minutes post-injection in situ continuously and comprised 2D T1 Weighted images for a certain time course. Details of the sequence were as follow: Bandwidth 20wHz, field of view 100×50 (cm2), matrix size 256×128, 20 coronal slices, slice thickness 1mm, TE= 1.68ms, TR= 80.02ms, FA= 45°, repetition 250, time resolution 7.68s. The histochemical studies were carried out six minutes after injection and the tissue of kidney was collected, sectioned, and stained with chlorophosphonazo III (CPN III).RESULTS
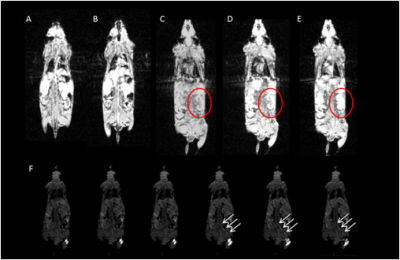
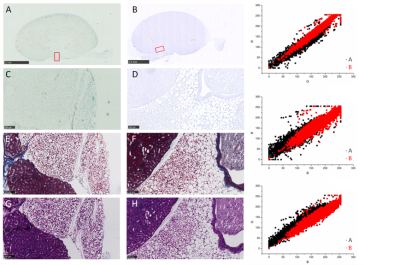
PAA-Gd performs higher longitudinal relaxivity (Fig.1 C, D) and lower cytotoxcity (Fig.1 B) compared with Magnevist (Gd-DTPA). DCE results showed the change of signal intensity of ROIs along time series. Immediately after PAA-Gd injection via ISI at tarsal tunnel, the MR signal intensity went high in response of intense increase in local Gd3+ concentration because which resulted in decreased local T1 value. Due to the compactness and continuity of fasciae along the ankle and leg, the macromolecule compound PAA-Gd existed inside of interstitial stream with little leakage into blood as observed (Fig.2 C-F). Besides, further free diffusion of PAA-Gd is not detected from DCE images in injection plane. The accumulation of PAA-Gd at injection point led to high local signal intensity. And the inner pressure motivated the flow of macromolecule agent along the pathway shown in Fig.2 (F) , which took approximately one or two scan-time (7.68 sec). Chemogenic reaction between CPNIII and Gd3+ from pruple to green shows the agent-stained region green in ISI group (Fig.3 A) and the non-agent region purple in IVI group (Fig.3 B). The histopathological result performs consistency with DCE data.DISCUSSION
Because the high relaxivity of PAA-Gd, the DCE sequence can provide high contrast ROIs verses other regions in biological tissue with low dosage and also there would not be long TR that constrained time resolution for its utmost optimizing parameter to match local longitudinal relaxation. The transportation of PAA-Gd in ISI pathway works differently on its speed and routine whereas IVI method motivates the spread of most MR agents in whole body along arteries, veins and blood capillaries. The renal hilus and cortex contain loose connective tissue and adipose tissue, which prevents the macromolecule agent from mass leakage to vascular or to other organs. Lightened path from ankle to kidney and the high signal intensity at renal hilus and cortex will not reject the conjecture that a high efficient pathway starts from tarsal tunnel at ankle, goes by para-vascular pathway and terminates at kidney tissue via the interstitial stream built by fascia. And the PAA-Gd agent can detect the interstitial stream with high specificity and sensitivity in living body.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 61971151).References
1 Feng J, Wang F, Han X, et al. A “green pathway” different from simple diffusion in soft matter: Fast molecular transport within micro/nanoscale multiphase porous systems. Nano Research. 2014;7(3):434-442.
2 Han X, Li H, Hua W, et al. Fluid in the tissue channels of vascular adventitia investigated by AFM and TEM. Clinical Hemorheology and Microcirculation. 2017;67(2):173-182.
3 R.A. Jones, Soft condensed matter, IOP Publishing (2002).
4 P.G. de Gennes, Soft matter, Science 256(5056) (1992), 495–497.
5 Shi X, Zhu Y, Hua W, et al. An in vivo study of the biodistribution of gold nanoparticles after intervaginal space injection in the tarsal tunnel. Nano Research. 2016;9(7):2097-2109.
6 Hu N, Cao Y, Ao Z, et al. Flow behavior of liquid metal in the connected fascial space: intervaginal space injection in the rat wrist and mice with tumor. Nano Research. 2018;11(4):2265-2276.
7 Li, H. Y., Chen, M., Yang, J. F.,et al.Suonan, C. Fluid flow along venous adventitia in rabbits: Is it a potential drainage system complementary to vascular circulations? PLoS One 2012, 7, e41395.
8 Toru Chikui, Makoto Obara, ArjanW. Simonetti, et al. The Principal of Dynamic Contrast Enhanced MRI, the Method of Pharmacokinetic Analysis, and Its Application in the Head and Neck Region. Int J Dent. 2012;2012:480659.
Figures