1196
Novel phosphorous-based polymer for 31P-magnetic resonance imaging
Natalia Ziółkowska1,2, Ladislav Androvič3, Lucie Woldřichová3, Martin Vít1,4, David Červený1,5, Olga Šebestová Janoušková3, Richard Laga3, and Daniel Jirák1,2
1Site of Computed Tomography, Magnetic Resonance Imaging, and Clinical and Experimental Spectroscopy, Institute for Clinical and Experimental Medicine, Prague, Czech Republic, 2Institute of Biophysics and Informatics, First Faculty of Medicine, Charles University, Prague, Czech Republic, 3Institute of Macromolecular Chemistry, Czech Academy of Sciences, Prague, Czech Republic, 4Faculty of Mechatronics Informatics and Interdisciplinary Studies, Technical University of Liberec, Liberec, Czech Republic, 5Faculty of Health Studies, Technical University of Liberec, Prague, Czech Republic
1Site of Computed Tomography, Magnetic Resonance Imaging, and Clinical and Experimental Spectroscopy, Institute for Clinical and Experimental Medicine, Prague, Czech Republic, 2Institute of Biophysics and Informatics, First Faculty of Medicine, Charles University, Prague, Czech Republic, 3Institute of Macromolecular Chemistry, Czech Academy of Sciences, Prague, Czech Republic, 4Faculty of Mechatronics Informatics and Interdisciplinary Studies, Technical University of Liberec, Liberec, Czech Republic, 5Faculty of Health Studies, Technical University of Liberec, Prague, Czech Republic
Synopsis
Our work is focused on the development and investigation of novel polymer-based contrast agent for 31P magnetic resonance (31P MR). The proposed diagnostics is a methacrylate-type polymer containing a phosphorothioate group (p(TMPC)), which causes a significant chemical shift of phosphorus, making it easily distinguishable from other biological phosphorus components, and is thus easily traceable by 31P MRI. Results from 31P MR imaging, spectroscopy and relaxometry obtained at 4.7T show very good MR properties, thus p(TMPC) represent a very promising diagnostic tool for in vivo use.
Introduction
The main obstacle in application of phosphorus-containing MR contrast agents for in vivo measurements is high natural 31P signal from the surrounding tissue. We would like to address this problem by presenting a metal-free probe based on phosphorus-containing polymer of methacrylate-type (p(TMPC)), which is traceable by 31P MRI and MRS, because its signal can be reliably separated from the others related to biological phosphorus-containing compounds. This is possible due to the presence of phosphorothioate group, ensuring large chemical shift of the polymer signal in the 31P MR spectra from Larmor frequencies of the tissue. This proof of principle study is comparing two polymers with the same backbone, but one bearing a phosphoester group (p(MPC)), which is commonly present in biological phosphorus-containing compounds, and the other containing a phosphorothioate group (p(TMPC)), which, in turn, is extremely rare in living organisms1. High phosphorus concentration in the p(TMPC) polymer together with the high frequency shift compared to the p(MPC) probe would enable imaging with sharp 31P signal boundary of our area of interest in the organism. We visualized 31P polymers at 4.7T scanner, using surface 1H/31P custom-made dual coil intended for small laboratory animals.Methods
Both presented polymers – poly[O-(2-(methacryloyloxy)ethyl) O-(2-(trimethylamoniumyl)ethyl) phosphorothioate] (p(TMPC)) and 2-(methacryloyloxy)ethyl 2-(trimethylammonio)ethyl phosphate p(MPC) were synthesized by controlled radical polymerization technique (RAFT) of the corresponding zwitterionic monomer. The phosphorothioate group (P=S) in the p(TMPC) polymer provides a chemical shift different from the 31P MR signal of the naturally occurring phosphorus-containing compounds, represented herein by the reference p(MPC) probe with a phosphoester group (P=O). MR imaging and spectroscopy were obtained on 4.7T scanner using home-made 1H/31P RF surface coil, which is tuned for both 1H and 31P nuclei, so that anatomical reference acquired by 1H MR could be obtained. Firstly, 1H MR imaging (RARE, repetition time/echo time TR/TE=2500/12ms; field of view FOV=10cm; scan time ST=1min) was applied in three planes for probes localization. MR properties were than assessed by 31P MRS/MRI measurement of phantom (cP=100mmol/L; V=1.4ml in H2O). Single pulse sequence was used for obtaining 31P T1 relaxation times (TR=200–4000ms; ST=30min–10h) and for frequency offset assessment of both contrast agents with different scan times (TR=200ms; ST=20s–3h). 31P relaxation times T2 were measured using Carr–Purcell–Meiboom–Gill (CPMG) spin lock sequence (TE=2–1200ms; ST=1h 23min). For 31P MRI chemical shift imaging (CSI) sequence was optimized (TR=500ms, ST=15min–3h, FOV=4.0cm; resolution 2.5x2.5x0.02mm3). 1H/31P MRI overlapping and 31P image processing were obtained using imageJ software. MR spectra were evaluated using homemade MATLAB script. 1H relaxation times T1 and T2 were obtained using 1.5T relaxometer (cP 10–100 mmol/L; V=240 µl in H2O). AlamarBlue Assay was used for testing cytotoxicity of the polymer on primary human fibroblasts (HF) and rat mesenchymal stem cells (rMSC).Results
1H MR T1/T2relaxation times (2510.0ms/2067.2ms; cP=100 mmol/L), were found to be close to those from clear water. 31P MR T1/T2 relaxation times (2018.3/119.9ms; cP=100mmol/L) are adequate for further MR experiments. 31P MR spectroscopy showed a chemical shift of 56.07ppm between the peaks from polymers with phosphoester and phosphorothioate groups, respectively (Fig.1). This shift made it possible to acquire 31P images from both phantoms separately during one CSI measurement by frequency selection (Fig.2). Signal-to-noise ratio calculated from 31P spectra results in 13.1–195.1 (ST=2min–3h) for probe with phosphorothioate bond and 12.5–163.7 for its reference. Imaging analyses results in SNR of 6.3–13.6 (ST=15min–3h) and 3.1–5.4, respectively. Higher SNR in both 31P MRS/MRI obtained from probe with phosphorothioate group favours it over the reference. Cytotoxicity testing confirms that the polymer is not significantly influencing cells viability even at the concentration reaching 10mg/ml.Discussion
High phosphorus concentration and SNR obtained from small volume within a short scan time is beneficial for further application in various in vivo animal models, where low probe signal is gained and short acquisition time is needed. Large chemical shift of the probe from frequencies of biological 31P spectra gives a unique possibility to distinguish its signal even at in vivo conditions. Our preliminary imaging and spectroscopy results, together with excellent cell viability, indicate that p(TMPC) polymer could serve as an efficient theranostic agent. Results obtained from 1H MR relaxometry shows that the polymer did not significantly affect water relaxation times, thus reference 1H images will be free of artefact originated from the polymer.Conclusion
We present a proof of principle results of a novel 31P MR of phosphorus-containing contrast agent based on chemical shift. The MR experiments proved high sensitivity of p(TMPC) probe for 31P MRI/MRS and ability to differentiate its 31P signal from biological phosphorus signals.Acknowledgements
The authors acknowledge financial support from the Ministry of Health of the Czech Republic (grant # NU20-08-00095). The study was also supported by the Charles University, GA UK No 358119; Charles University, First Faculty of Medicine; Institute for Clinical and Experimental Medicine IKEM, IN00023001; Ministry of Education of the Czech Republic through the SGS project no. 21332/3012 of the Technical University of Liberec.References
1. Petkowski JJ, Bains W, Seager S. Natural Products Containing 'Rare' Organophosphorus Functional Groups. Molecules. 2019;24(5).
Figures
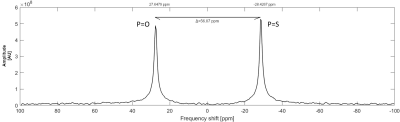
Fig.1 31P MR spectra measured at 4.7T scanner using
1H/31P RF surface coil. Single-pulse sequence (TR=200ms; ST=3h)
was used for the measurement and spectra from both p(TMPC) and p(MPC) polymers (cP=100mmol/L; V=1.4ml in H2O) were
obtained during one measurement. Peak on the left with slightly lower amplitude
represents a polymer with a phosphoester group (P=O) with SNR=163.7; peak on the right represents
a polymer with a phosphorothioate group (P=S)
with SNR=195.1. The chemical shift between the peaks is 56.07ppm.

Fig. 2 MRI
measured at 4.7T scanner using 1H/31P RF surface coil. Phantoms (cP=100mmol/L;
V=1.4ml in H2O) positioning
is visible on 1H MR image (RARE sequence; ST=1min; coronal plane) with
probes containing a phosphoester (P=O) group on the left and a phosphorothioate
(P=S) group on the right with water reference between them (A). Next, 31P
MRI (CSI
sequence; ST=3h) of polymers (B: P=O; C: P=S) are presented. In
the last image, overlapped 1H/31P MR images are shown (D);
31P signal is highlighted by green colour. SNR resulting from 31P
MR images is 5.4 for P=O polymer and 13.6 for P=S polymer.