1192
Microfluidic preparation of liposomal hydrogel microbeads containing CT agents (CT-lipobeads) to longitudinally monitor pH using CEST MRI1Department of Biomedical Engineering, City University of Hong Kong, Hong Kong, Hong Kong, 2Department of Pathology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, Hong Kong, 3State Key Laboratory of Liver Research, The University of Hong Kong, Hong Kong, Hong Kong, 4City University of Hong Kong Shenzhen Research Institute, Shen Zhen, China, 5Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Iodinated CT contrast agents(CA) had been investigated as CEST contrast agent while high dose of CA and quick clearance could limit their potential for monitoring pH longitudinally. Here, we aim to introduce CT-lipobeads to address this issue. The CT-lipobeads showed 10% CEST contrast and good pH dependency at pH 6.0-7.0, which is sensitive to distinguish hypoxic medium from normoxic medium. Moreover, CT-lipobeads generated CEST contrast of 9% in the subcutaneous compartment, and contrast remained constant over a week. These findings demonstrated a robust approach for fabrication of CT-lipobeads with decent CEST contrast for longitudinal pH imaging.
Introduction
Iodinated CT contrast agents had been exploited as CEST contrast agents for pH detection both in preclinical and in clinic and showed decent precision(0.07 pH unit).(1-5) However, high dose of contrast agents(CA), which could be harmful to organs, such as kidneys,(6) and quick clearance of CA could limit their potential for longitudinal pH monitoring. Incorporating CA into liposomal microbeads could be an alternative. We had demonstrated that the design of liposomal microbeads could provide a high local concentration of CA in vivo with stable contrast over a month using CEST MRI.(7) Microfluidic synthesis and assembly offered a facile approach to the continuous production of microbeads with unprecedent control over their sizes, shapes, and morphologies.(8-10) In this study, we aim to fabricate microbeads with decent CEST contrast and pH sensitivity, and minimal release of contrast agents for longitudinal monitoring. We developed a robust microfluidic device for the fabrication of CT-lipobeads, which Iodine CT contrast agents were loaded into liposomes before forming microbeads. We studied their CEST contrast at 3T. The results showed that this microfluidic approach generated microbeads at a few tens of microns and the CT-lipobeads possessed distinctive CEST contrast at 4.2 ppm, pH sensitivity at pH 6.0-7.0 and minimal release of CA over 3 weeks. These unique properties could facilitate the clinical translation of Iodinated CA in longitudinal pH monitoring in vivo.Methods
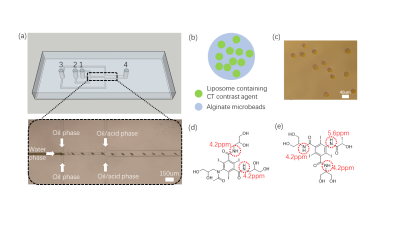
Liposomes were prepared using thin film hydration method.(11) Microfluidic devices were fabricated by soft lithography and replica modeling of PDMS.(12,13) The design and preparation were shown in Fig. 1. Phantoms were imaged on a horizontal bore 3T Bruker BioSpec system at 37 oC. The CEST MRI sequence was a continuous-wave (CW) saturation module, followed by the rapid acquisition with refocused echoes (RARE) as a readout module. MRI parameters as following saturation parameters: B1 were 0.6, 0.8, 1.2, 1.6, 2.0 and 3.0uT, Tsat=3000ms and RARE factor = 32. CEST contrast was calculated by applying Lorentzian fitting on Z spectra. 150ul microbeads were mixed with 300ul HM(pH6.8), NM(pH 7.1) and FM(pH 7.2). 300ul CT-lipobeads were subcutaneously injected to ICR mouse under anesthesia.Results and discussion
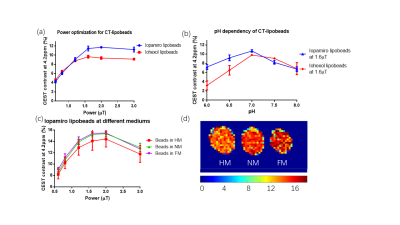
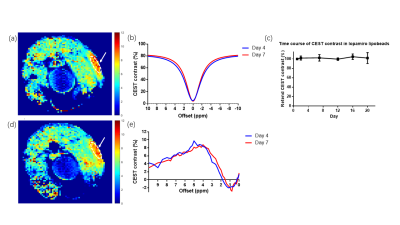
The average size of prepared CT-lipobeads was 38um (Fig. 1c), which could favor administration via injection. The CEST contrast increased as the B1 increases from 0.6uT to 2.0uT, and it reached a maximum contrast of 11.7% for Iopamiro CT-lipobeads and 9.7% for Iohexol CT-lipobeads at 4.2 ppm, in the presence of amide protons of CT contrast agents(1,14-18) (Fig. 2a). CEST contrast at 4.2ppm increased with pH and reach maximum at pH 7.0, then decreased(Fig 2b), likely because of the broadening of the amide resonances.(16,18) CEST contrast difference of Iopamiro and Iohexol CT-lipobeads at 6.5-7.0 pH were 1.5% and 3.3% at 1.6mT, respectively, which indicates the Iohexol CT-lipobeads is more sensitive at this pH range. This is due to the different exchange rate at pH 6.5 from that at pH 7.0. The Iopamiro CT-lipobeads showed 14.1%, 15.2% and 15.4% CEST contrast at 4.2 ppm in hypoxic medium, normoxic medium and fresh medium, respectively(Fig. 2c). Iopamiro CT-lipobeads showed a 8.4% decrease in CEST contrast at 4.2 ppm, which could indicate an acidic tumor microenvironment. The CEST contrast at 4.2ppm remained constant after 3-week incubation in saline(Fig. 3c). After injection into the subcutaneous region of mice, CT-lipobeads generated 8.7% and 8.1% CEST contrast at 4.2ppm at 1.2uT on day 4 and day 7, respectively(Fig. 3a, 3b and 3d). This pilot in vivo study demonstrated that CT-lipobeads could be valuable for longitudinal pH detection. We are now optimizing the platform to generate CT-lipobeads with higher CEST contrast e.g. replacing Ca2+ with Ba2+ or adding chitosan coating could further enhance the retention of contrast agents(19). Moreover, the feasibility of detecting pH changes in tumor microenvironment are underway.Conclusions
We demonstrated the feasibility of fabricating pH sensitive CEST microbeads using microfluidic approach. CEST contrast of CT-lipobeads showed pH dependency and they could distinguish hypoxic medium from normoxic medium. Stable CEST contrast was observed for 3 weeks in vitro. The CEST contrast was ~9 % after injection into the subcutaneous compartment of mice for a week, which support the possibility of longitudinal in vivo imaging. We believe this platform can provide an alternative way to monitor pH during the course of treatment.Acknowledgements
This study was supported by Research Grants Council: 11102218; City University of Hong Kong: 7005210, 9680247, 9667198 and 6000660; National Natural Science Foundation of China: 81871409.References
1.Aime S, Calabi L, Biondi L, De Miranda M, Ghelli S, Paleari L, Rebaudengo C, Terreno E. Iopamidol: Exploring the potential use of a well-established x-ray contrast agent for MRI. Magnetic resonance in medicine 2005;53(4):830-834.
2. Chen LQ, Howison CM, Jeffery JJ, Robey IF, Kuo PH, Pagel MD. Evaluations of extracellular pH within in vivo tumors using acidoCEST MRI. Magnetic resonance in medicine 2014;72(5):1408-1417. 3. Moon BF, Jones KM, Chen LQ, Liu P, Randtke EA, Howison CM, Pagel MD. A comparison of iopromide and iopamidol, two acidoCEST MRI contrast media that measure tumor extracellular pH. Contrast media & molecular imaging 2015;10(6):446-455.
4. Longo DL, Bartoli A, Consolino L, Bardini P, Arena F, Schwaiger M, Aime S. In Vivo Imaging of Tumor Metabolism and Acidosis by Combining PET and MRI-CEST pH Imaging. Cancer Res 2016;76(22):6463-6470.
5. Anemone A, Consolino L, Conti L, Irrera P, Hsu MY, Villano D, Dastru W, Porporato PE, Cavallo F, Longo DL. Tumour acidosis evaluated in vivo by MRI-CEST pH imaging reveals breast cancer metastatic potential. Br J Cancer 2020.
6. Cheng W, Zhao F, Tang CY, Li XW, Luo M, Duan SB. Comparison of iohexol and iodixanol induced nephrotoxicity, mitochondrial damage and mitophagy in a new contrast-induced acute kidney injury rat model. Arch Toxicol 2018;92(7):2245-2257. 7. Chan KW, Liu G, Song X, Kim H, Yu T, Arifin DR, Gilad AA, Hanes J, Walczak P, van Zijl PC, Bulte JW, McMahon MT. MRI-detectable pH nanosensors incorporated into hydrogels for in vivo sensing of transplanted-cell viability. Nature materials 2013;12(3):268-275.
8. Tumarkin E, Kumacheva E. Microfluidic generation of microgels from synthetic and natural polymers. Chem Soc Rev 2009;38(8):2161-2168.
9. Zhao Q, Cui H, Wang Y, Du X. Microfluidic Platforms toward Rational Material Fabrication for Biomedical Applications. Small 2020;16(9):e1903798.
10. Alkayyali T, Cameron T, Haltli B, Kerr RG, Ahmadi A. Microfluidic and cross-linking methods for encapsulation of living cells and bacteria - A review. Anal Chim Acta 2019;1053:1-21.
11. Chan KW, Yu T, Qiao Y, Liu Q, Yang M, Patel H, Liu G, Kinzler KW, Vogelstein B, Bulte JW, van Zijl PC, Hanes J, Zhou S, McMahon MT. A diaCEST MRI approach for monitoring liposomal accumulation in tumors. Journal of controlled release : official journal of the Controlled Release Society 2014;180:51-59.
12. Xia Y, Whitesides GM. Soft lithography. Angewandte Chemie International Edition 1998;37(5):550-575.
13. Ren J, Li J, Li Y, Xiao P, Liu Y, Tsang CM, Tsao S-W, Lau D, Chan KW, Lam RH. Elasticity-modulated microbeads for classification of floating normal and cancer cells using confining microchannels. ACS Biomaterials Science & Engineering 2019.
14. Zu Z, Li H, Jiang X, Gore JC. Spin‐lock imaging of exogenous exchange‐based contrast agents to assess tissue pH. Magnetic resonance in medicine 2018;79(1):298-305.
15. Longo DL, Busato A, Lanzardo S, Antico F, Aime S. Imaging the pH evolution of an acute kidney injury model by means of iopamidol, a MRI-CEST pH-responsive contrast agent. Magnetic resonance in medicine 2013;70(3):859-864.
16. Longo DL, Dastru W, Digilio G, Keupp J, Langereis S, Lanzardo S, Prestigio S, Steinbach O, Terreno E, Uggeri F, Aime S. Iopamidol as a responsive MRI-chemical exchange saturation transfer contrast agent for pH mapping of kidneys: In vivo studies in mice at 7 T. Magnetic resonance in medicine 2011;65(1):202-211.
17. High RA, Ji Y, Ma YJ, Tang Q, Murphy ME, Du J, Chang EY. In vivo assessment of extracellular pH of joint tissues using acidoCEST-UTE MRI. Quant Imaging Med Surg 2019;9(10):1664-1673.
18. Longo DL, Michelotti F, Consolino L, Bardini P, Digilio G, Xiao G, Sun PZ, Aime S. In Vitro and In Vivo Assessment of Nonionic Iodinated Radiographic Molecules as Chemical Exchange Saturation Transfer Magnetic Resonance Imaging Tumor Perfusion Agents. Invest Radiol 2016;51(3):155-162.
19. Bhopatkar D, Anal AK, Stevens WF. Ionotropic alginate beads for controlled intestinal protein delivery: effect of chitosan and barium counter-ions on entrapment and release. J Microencapsul 2005;22(1):91-100.
Figures