1164
Resolving fold-over artefacts for Reduced Field-of-View Parallel Imaging with Cartesian Sampling
Sen Jia1, Zhilang Qiu1,2, Lei Zhang1, Haifeng Wang1, Xin Liu1, Hairong Zheng1, and Dong Liang1
1Paul C. Lauterbur Research Center for Biomedical Imaging, Shenzhen Institutes of Advanced Technology, Shenzhen, China, 2University of Chinese Academy of Sciences, Beijing, China
1Paul C. Lauterbur Research Center for Biomedical Imaging, Shenzhen Institutes of Advanced Technology, Shenzhen, China, 2University of Chinese Academy of Sciences, Beijing, China
Synopsis
Equipping the existing parallel imaging methods such as ESPIRiT and SPIRiT with a full field-of-view (FOV) calibration could resolve the fold-over artefacts induced by reducing the imaging FOV to be smaller than the object size. Full-FOV images could be reconstructed by accurately resolving the aliased components in image space, or by reconstructing the kspace at a finer sampling interval corresponding to full-FOV. Both approaches requires a separate full-FOV calibration data which could be acquired efficiently. Reduced FOV Parallel imaging methods with full-FOV calibration may provide an alternative approach to treat the common FOV aliasing problem in practice.
Introduction
The Nyquist-Shannon sampling theorem demands the MR imaging field-of-view (FOV) be larger than the imaged object to avoid fold-over artifacts1,2,3. In practice, the imaging FOV may be reduced intentionally to be smaller than the object size to increase imaging speed without scarifying imaging resolution, e.g., in isotropic high-resolution whole-brain imaging4. Fold-over/aliasing artifacts would occur at the image boundary, further limit the choice of subsequent parallel imaging methods and their acceleration performance, such as increased noise amplification2 and potential central aliasing artifacts3. This work proposes extensions to the existing parallel imaging algorithms5,6 to resolve the boundary fold-over artifacts and reconstruct full-FOV images for reduced FOV parallel imaging.Methods
ESPIRiT5 models the FOV fold-over of reduced FOV parallel imaging by using multiple coil sensitivity maps (CSM) correspond to multiple eigenvectors for eigenvalue “=1”. This model could reconstruct reduced FOV images without central aliasing artifacts but still suffers from residual boundary fold-over artifacts5. We propose to firstly estimate a full-FOV ESPIRiT map from calibration data (ACS) acquired separately at a finer sampling interval than the imaging scan; then create multiple maps according to the folder-over process of reduced FOV imaging (Figure 1). Finally, soft-SENSE reconstruction 5 using the new multiple maps could resolve the aliasing components and reconstruct a full-FOV image. The proposed ESPIRiT reconstruction with full-FOV calibration has a similar scan and reconstruction efficiency as the original ESPIRiT. Full-FOV calibration could also apply to kspace parallel imaging methods such as SPIRiT and GRAPPA as illustrated in Figure 2. GRAPPA or SPIRiT kernels estimated from full-FOV ACS could reconstruct the full-FOV kspace from reduced FOV imaging data by direct convolution or iterative optimization.In-vivo experiments were IRB approved with informed consent obtained.All scans were performed on a 3T scanner (UIH uMR 780, China) with a 32-channel head coil. (1) High-resolution whole-brain imaging was performed on a 26 years old male healthy volunteer using a T1 weighted 3D MATRIX sequence (sagittal, non-selective excitation, 0.6 mm3 isotropic resolution, TE/TR = 8.8/800 ms). The imaging FOV in the phase and partition encoding directions were intentionally reduced to 178 (AP, anterior-posterior) x 152 (LR, left-right) mm2. Separate ACS (24x24) was acquired with full FOV being 220x206 mm2 for whole-brain coverage. The image size reduced from 366x342 to 296x252 would benefit both the scan and reconstruction efficiency, especially when iterative reconstruction was utilized. The scan was further accelerated by 2x2 uniform undersampling and took 4 minutes. (2) Another two 3D scans were performed on a 30 years old female healthy volunteer at an isotropic resolution of 0.6 mm3. The FOV along the LR direction was reduced from 210 mm to 144 mm. The two scans were accelerated by 2x2 uniform and 5-fold variable density Poisson-disc undersampling, respectively. Full-FOV ACS of size 24x24 was acquired with FOV being 220x210 mm2. (3) A head-shaped phantom was scanned by 3D gradient echo sequence twice using axial imaging orientation (FOV=240(LR)x240(AP)x64(HF) mm3, resolution=1 mm3 , TR/TE=11.8/4.5 ms), one with 100% slice oversampling (TA=6 min), while the other without oversampling (TA=3 min). A separate full-FOV ACS calibration of size 24x24 was acquired with 100% slice oversampling and took about 2 sec. All accelerated datasets were reconstructed by ESPIRiT/SPIRiT with reduced- or full-FOV calibration respectively. All iterative reconstruction utilized sparsity regularization with L1 weights manually optimized and 120 iterations. All algorithms were implemented in MATLAB and run on a workstation with two 40-cores CPUs with 256GB memory.
Results
Figure 3 compared the proposed four maps created from the full-FOV ESPIRiT map with the four maps estimated directly from reduced FOV ACS by ESPIRiT with the eigenvalue larger than 0.95. Soft-SENSE reconstruction using the multiple maps created from full-FOV CSM could accurately resolve the aliased pixels and reconstruct a full-FOV image without boundary fold-over artifacts.Equipping the image-domain ESPIRiT and k-space SPIRiT with full-FOV calibration could achieve the full-FOV reconstruction of reduced FOV imaging data, as demonstrated in Figure 4. Moreover, the proposed full-FOV reconstruction scheme was compatible with arbitrary sampling schemes and sparsity regularization. The computational complexity of the proposed extension to ESPIRiT was similar to the original ESPIRiT (24sec vs. 28sec). However, the computational burden of the proposed extension to SPIRiT would increase since the matrix size of the unknown increased from reduced FOV to full-FOV (80sec vs. 140sec).
In Figure 5, the axial 3D scan without slice oversampling suffered from fold-over artifacts due to the imperfect slice-selective excitation profile. Using the proposed GRAPPA or ESPIRiT reconstruction with full-FOV calibration, the fold-over artifacts could be resolved. This might provide an alternative approach to avoid fold-over artifacts for slice-selective 3D imaging, if acquiring separate full-FOV ACS is more efficient than slice oversampling.
Discussion
By equipping the existing parallel imaging methods with separately acquired full-FOV calibration data, the FOV aliasing from reduced FOV parallel imaging could be accurately resolved, and full-FOV images could be reconstructed with similar scan and reconstruction efficiency. Reduced FOV parallel imaging with full-FOV calibration may provide an alternative approach to deal with the common FOV aliasing problem in practice7. One potential limitation of the proposed methods is that inconsistency may occur between imaging scan and separate full-FOV calibration scan due to motion.Acknowledgements
This work is supported by the State Key Program of the National Natural Science Foundation of China (Grant No. 81830056), the National Natural Science Foundation of China (Grant No. 81801691), the National Key R&D Program of China (2017YFC0108802 and 2017YFC0112903), and Key Laboratory for Magnetic Resonance and Multimodality Imaging of Guangdong Province.References
- Hamilton J, Franson D, Seiberlich N. Recent advances in parallel imaging for MRI. Progress in Nuclear Magnetic Resonance Spectroscopy 2017, 101:71-95.
- Griswold MA, Kannengiesser S, Heidemann RM, Wang J and Jakob PM. Field‐of‐view limitations in parallel imaging. Magn Reson Med 2004, 52: 1118-1126.
- Goldfarb JW. The SENSE ghost: Field‐of‐view restrictions for SENSE imaging. J Magn Reson Imaging 2004, 20: 1046-1051.
- Jia S, Zhang L, Ren L, etc. Joint Intracranial and Carotid Vessel Wall Imaging in 5 minutes using Compressed Sensing accelerated DANTE-SPACE. Eur Radiol 2019, 30:119-127.
- Uecker M, Lai P, Murphy MJ, etc. ESPIRiT—an eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn Reson Med 2014, 71: 990-1001.
- Lustig M and Pauly JM. SPIRiT: Iterative self‐consistent parallel imaging reconstruction from arbitrary k‐space. Magn Reson Med 2010, 64: 457-471.
- Sandino, CM, Lai, P, Vasanawala, SS, Cheng, JY. Accelerating cardiac cine MRI using a deep learning-based ESPIRiT reconstruction. Magn Reson Med. 2020; 85: 152– 167.
Figures
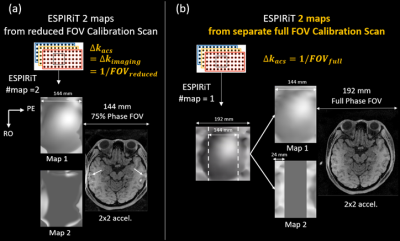
Figure 1. Multiple maps modelling
the aliasing of reduced field-of-view imaging could be created from full-FOV ESPIRiT
CSM estimated from full-FOV calibration scan, and used in soft-SENSE reconstruction to reconstruct full-FOV image without fold-over artifacts.
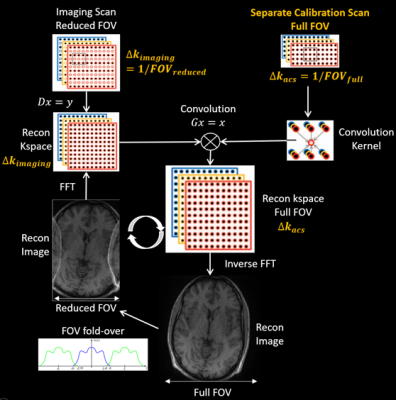
Figure 2. The image aliasing of
reduced field-of-view imaging could be resolved by parallel imaging reconstruction
of a full-FOV k-space using SPIRiT or GRAPPA with kernel estimated from full-FOV calibration scan
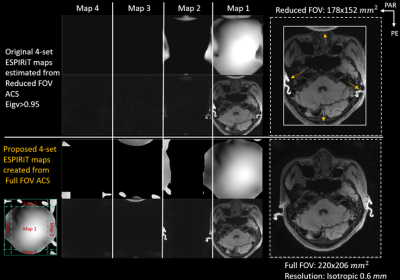
Figure 3. Full-FOV
reconstruction of reduced FOV parallel imaging along two phase encoding
directions with 2x2 downsampling by proposed ESPIRiT 4 maps created from full-FOV
calibration scan
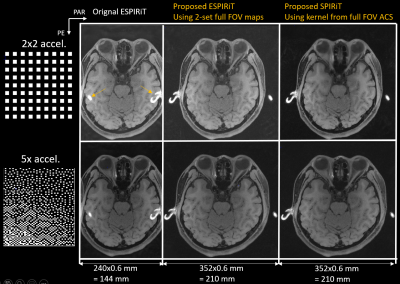
Figure 4. Full-FOV
reconstruction of reduced FOV parallel imaging datasets accelerated by different sampling patterns using proposed ESPIRiT and SPIRiT reconstruction with full-FOV calibration
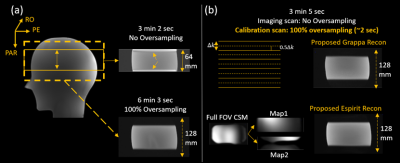
Figure 5. Field-of-view fold-over artifact
induced by imperfect slice-selective excitation profile could be resolved by
reduced FOV parallel imaging reconstruction with full-FOV calibration