1160
Accelerated 3D Myelin Water Imaging using Joint Parallel Imaging and Variable Splitting Network1Department of Electrical & Electronic Engineering, Yonsei Univ., Seoul, Korea, Republic of, 2Department of Radiology, Stanford Univ., Stanford, CA, United States
Synopsis
Myelin water imaging using multi-cho GRE (mGRE) requires long acquisition times. In this study, we explored a multi-step reconstruction method using both advanced parallel imaging and deep learning, which can utilize joint information between the multi-echo images to further accelerate the acquisition. The proposed method shows acceptable image quality with improved quantitative values compared to conventional methods. The proposed method can achieve high acceleration factors for mGRE based 3D myelin water imaging.
Introduction
Myelin water imaging (MWI) using multi-echo GRE (mGRE) requires long acquisition times. Acceleration for 3D mGRE acquisition has recently been achieved using parallel imaging (PI) algorithms. However, these algorithms typically allow low acceleration factor (R) of about only up to 2 for MWI due to the high sensitivity of the myelin estimation routine with respect to noise/artifacts1,2. Residual artifacts in the reconstructed mGRE images especially at the early echoes seriously influence the reconstructed MWI3.In this study, to overcome these limitations, we explored a multi-step reconstruction method which can utilize the joint information between the multi-echo images to further accelerate mGRE acquisition. In the initial step, a joint parallel imaging method named joint low-rank modeling of local k-space neightborhoods (JLORAKS)4 is performed to jointly reconstruction across echoes. Next, the remaining residual artifacts are subsequently reduced using a joint variable splitting network (JVS-net).
Method
[Data acquisition]All in-vivo data were acquired on a clinical 3T MRI scanner (Magnetom Tim Trio; Siemens Medical Solution, Erlangen, Germany)
3D mGRE scan protocol for full acquisition :
matrix size=128x128x72, resolution=2x2x2mm3, 16 coils, TR=46ms, 31 echo times (TE1=1.7ms, TE31=34.7ms, echo spacing=1.1ms), flip angle=20˚ and scan time=9:13min
[Reconstruction algorithm]
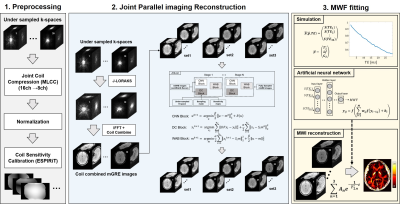
The reconstruction algorithm is shown in Figure 1. Retrospective undersampling process of the fully sampled 3D MR data in the first (R1) and second (R2) phase encoding line directions (R=R1*R2) were performed. All experiments used 20 auto-calibration lines, 8 compressed coils using multi-linear singular value decomposition8. Virtual coil augmentation was used to provide additional spatial encoding. Reconstructed k-space for each coils were combined using coil sensitivity maps estimated from ESPIRiT9. Next, the resulting images were applied to JVS-net, pretrained on data obtained from 10 subjects. For the JVS-net, the original variable splitting network10 was modified and extended to apply the joint reconstruction scheme. Moreover, we utilized partially joint information for JVS-net in order to achieve more elaborate reconstruction. We tested the method on one in-vivo data which was left out from the training set. Finally ANN-based MWI reconstruction method was applied for MWI estimations5.
Results
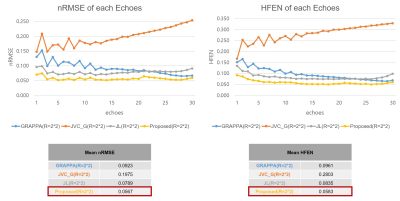
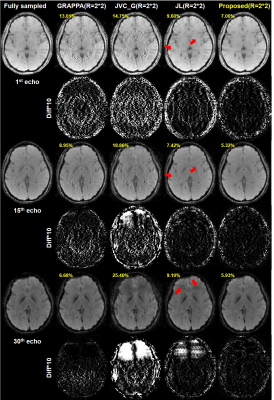
Quantitative comparison of nRMSE and HFEN values between conventional GRAPPA6, JVC-GRAPPA7, JLORAKS versus the proposed method are shown in Figure 2. In the situation where the acceleration factor R=2*2, the proposed method shows the lowest nRMSE and HFEN values for all echoes.Comparative results of reconstructed mGRE images with difference maps are shown in Figure 3. The improved performance of the proposed method can be observed. Residual artifacts (red arrows) in the JLORAKS method have been reduced on the images reconstructed from the proposed method. Also the nRMSE values showed reduced values.
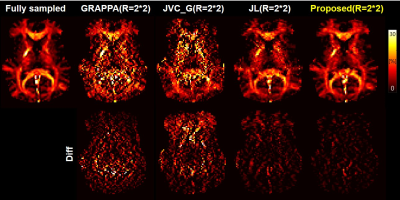
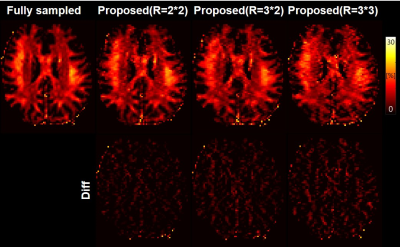
MWIs of the different methods are shown in Figure 4. The improved quality of MWIs generated by the proposed method can be seen. MWIs of the proposed method by applying further acceleration factors (R=3*2, R=3*3) are shown in Figure 5. Unacceptable images are created when R=3*3.
Discussion and Conclusion
In this study, we demonstrated accelerated 3D MWI through joint PI reconstruction with deep learning network. The proposed method showed 1) lower nRMSE, HFEN values compared with conventional methods and 2) high similarity with fully-sampled reconstructed images especially in the early echo images critical for myelin water estimation. Consequently, the reconstructed MWIs of the proposed method show similar results with MWIs from fully-sampled reference.Acknowledgements
This work was supported by the National Research Foundation of Korea(NRF) grant funded by the Korea government(MSIT) (NRF-2019R1A2C1090635)References
1. Chen H, et al. "Compressed Sensing CPMG with Group-Sparse Reconstruction for Myelin Water Imaging." Magnetic resonance in medicine 71.3 (2014): 1166-1171.
2. Dvorak A, et al. "Multi-spin echo T2 relaxation imaging with compressed sensing (METRICS) for rapid myelin water imaging." Magnetic resonance in medicine 84.3 (2020): 1264-1279.
3. Quan C, et al. "Accelerating Myelin Water Quantification using Tensor Dictionary Learning with Low-Rank Plus Sparse Regularization." IEEE Transactions onMedical Imaging (2020).
4. Bilgic B, et al. "Improving parallel imaging by jointly reconstructing multi‐contrast data." Magnetic resonance in medicine 80.2 (2018): 619-632.
5. Soozy J, et al. "Artificial neural network for multi-echo gradient echo-based myelin water fraction estimation." Magnetic resonance in medicine 85.1 (2020): 380-389.
6. Griswold MA, et al. "Generalized autocalibrating partially parallel acquisitions (GRAPPA)." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 47.6 (2002): 1202-1210.
7. Haifeng W, et al. "Improving GRAPPA reconstruction using joint nonlinear kernel mapped and phase conjugated virtual coils" Physics in Medicine & Biology 64.14 (2019): 14NT01.
8. Biyik E, et al. "Reconstruction by calibration over tensors for multi‐coil multi‐acquisition balanced SSFP imaging." Magnetic resonance in medicine 79.5 (2018): 2542-2554.
9. Uecker M, et al. "ESPIRiT—an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA." Magnetic resonance in medicine 71.3 (2014): 990-1001.
10. Jinming D, et al. "VS-Net: Variable splitting network for accelerated parallel MRI reconsturction." International Conference on Medical Image Computing and Computer-Assisted Intervention. Springer, Cham (2019): 713.722.
Figures