1154
Reduced Field of View Parallel Imaging with Wave Encoded k-Space Trajectory1Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2University of Chinese Academy of Sciences, Beijing, China
Synopsis
Parallel imaging with wave encoded k-space trajectory can use most of the existing reconstruction methods such as SENSE, ESPIRiT and SPIRiT. However, these reconstruction methods are not suitable to reduced field-of-view (FOV) imaging scenario, where the imaging FOV is smaller than the object size, and severe aliasing artifacts remain in the reconstructed images. This work proposes to extend the existing reconstruction methods to treat the reduced FOV case, by accurately modeling and resolving the aliased components. Without increasing scan time, the artifact-free and full FOV images can be reconstructed by the proposed methods.
Introduction
Wave-CAIPI1 is a parallel imaging technique that utilizes the spatial variation of 3D coil sensitivity by causing aliasing in all three dimensions. It employs sinusoidal (wave) gradients to cause spreading aliasing along the readout (RO) direction, which can be characterized by the wave-point spread function (PSF). Parallel imaging with wave encoded k-space trajectory can use most of the existing reconstruction methods, such as SENSE2, ESPIRiT3 and SPIRiT4, by incorporating the wave-PSF in the forward model. However, with wave encoded k-space trajectory, these reconstruction methods cannot handle the reduced field-of-view (FOV) case, where the imaging FOV is smaller than the object size5, and severe aliasing artifacts remain in the reconstructed images. This work proposes to extend the existing reconstruction methods, ESPIRiT and SPIRiT (referred to as r2f-ESPIRiT and r2f-SPIRiT, respectively) to reconstruct artifact-free images in reduced FOV imaging scenario, with full FOV images additionally obtained without adding scan time.Methods
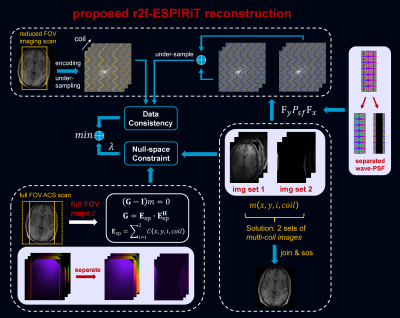
ESPIRiT models the fold-over induced by reduced FOV by using multiple eigenvector maps corresponding to eigenvalue “=1”. However, this model cannot model the aliasing correctly for wave encoded reduced FOV imaging, even a full FOV wave-PSF is considered. We propose to model the fold-over process accurately by using a full FOV coil sensitivity map in the proposed r2f-ESPIRiT. Similarly, the idea is extended to a k-space based reconstruction by using a full FOV auto-calibration in the proposed r2f-SPIRiT.Fig. 1 illustrates the proposed r2f-ESPIRiT reconstruction. The model uses 2 sets of coil sensitivity maps created from the primary set of ESPIRiT maps estimated from a full FOV auto-calibrating scan (ACS), according to the fold-over process caused by reduced FOV. The model also incorporates 2 sets of wave-PSF created from a full FOV wave-PSF. The proposed method can resolve the fold-over components, and reconstruct a full FOV image from the accelerated reduced FOV imaging scan.
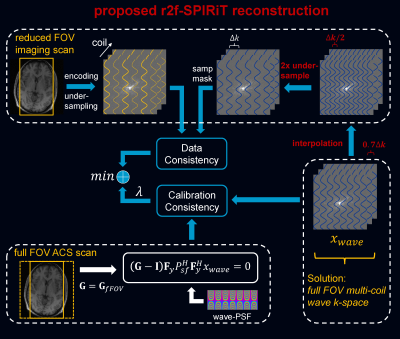
Fig. 2 shows the proposed r2f-SPIRiT reconstruction, where a convolution kernel estimated from a full FOV ACS is used, and a full FOV wave-PSF is provided as well. Although accelerated reduced FOV imaging data is acquired, the multi-coil wave k-space corresponding to a full FOV image is reconstructed, which can be de-convoluted by the wave-PSF and combined subsequently.
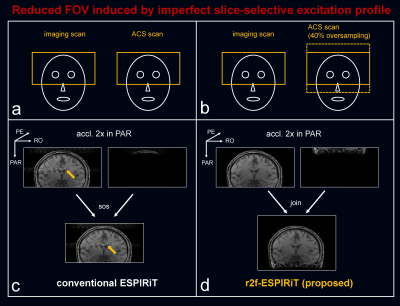
In vivo experiments were IRB approved with informed consent obtained. All scans were performed on a 3T uMR 790 scanner (United Imaging Healthcare, Shanghai, China). (1) The wave gradient of sinusoidal waveform was implemented in a 2D RF-spoiled gradient (GRE) sequence. A 2D imaging scan was performed (transversal, planar resolution=1×1 mm2, TR/TE=250/5 ms, FA=70 degree, slice thickness=5 mm, wave relative amplitude=1.5, cycle=8). The imaging FOV in the phase encoding (PE) direction was intentionally reduced to 136 mm (LR, left-right) to induce a reduced FOV, while it was 256 mm with full FOV in the readout (RO) direction. Separate ACS (48×48) was acquired with full FOV being 256 × 250 mm2. A retrospectively 3-fold acceleration was perform to the wave encoded reduced FOV imaging data. The proposed r2f-ESPIRiT reconstruction was performed, compared to the conventional ESPIRiT with 2 sets of maps. And, the proposed r2f-SPIRiT reconstruction applied to the same data, compared to conventional SPIRiT. (2) The Wave-CAIPI technique was implemented in a 3D GRE sequence. A 3D imaging scan was perform with slice-selective excitation and transversal orientation, but slice-oversampling was not used in the imaging scan to save time. A reduced FOV was induced by the imperfect slice-selective excitation profile in the head-foot (HF) direction. An acceleration of 1 (PE) × 2 (PAR) was conducted and inverse Fourier transform was performed along PE to create a series of separate 2D wave k-space of RO-PAR plane for the subsequent 2D reconstruction using the proposed methods. Wherein, 40% slice-oversampling was used to generate a full FOV ACS scan.
Results
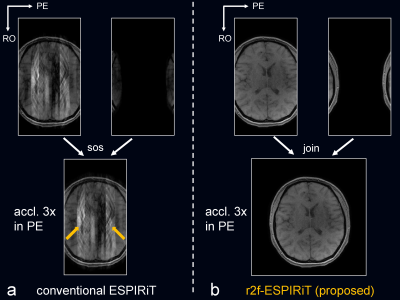
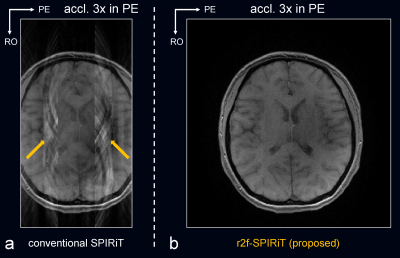
Fig. 3 compared the proposed r2f-ESPIRiT to conventional ESPIRiT, and Fig. 4 compared the proposed r2f-SPIRiT to original SPIRiT. Both the proposed methods can not only reconstruct artifact-free images, but also resolve the aliased components induced by reduced FOV. Fig. 5 shows the case of reduced FOV induced by imperfect slice-selective excitation profile. The fold-over artifacts can be resolved by the proposed methods, without adding scan time.Discussion
Although not commonly used, the reduced FOV scenario is required in some applications, e.g. in high-resolution whole-brain imaging6, to increase imaging speed and reconstruction efficiency. Also, it appears in slice-selective 3D imaging if slice-oversampling is not used to save time. The proposed methods in this work may provide an alternative solution to deal with these cases, without adding scan time. One potential limitation is that inconsistency may occur between imaging scan and separate full-FOV calibration scan due to motion. The proposed methods are demonstrated in Wave-GRE sequence1,7 currently, it would be tested on more sequences such as Wave-bSSFP8 and Wave-SPACE9,10 in our future work.Acknowledgements
Zhilang Qiu and Sen Jia contributed equally to this work.
This work was supported in part by the National Key R&D Program of China (No. 2017YFC0108802 and 2017YFC0112903), the Strategic Priority Research Program of Chinese Academy of Sciences (No.XDB25000000), the Pearl River Talent Recruitment Program of Guangdong Province (No.2019QN01Y986), the Shenzhen Peacock Plan Team Program (No.KQTD20180413181834876), the Chinese Academy of Sciences Engineering Laboratory for Medical Imaging Technology and Equipment (No.KFJ-PTXM-012), the Shenzhen Key Laboratory of Ultrasound Imaging and Therapy (No. ZDSYS201802061806314), the Natural Science Foundation of Guangdong Province (No.2018A0303130132) and the National Natural Science Foundation of China (No.61871373, No.81729003 and No.81901736). the State Key Program of the National Natural Science Foundation of China (Grant No. 81830056) and the Key Laboratory for Magnetic Resonance and Multimodality Imaging of Guangdong Province.
References
1. Bilgic B, Gagoski BA, Cauley SF, Fan AP, Polimeni JR, Grant PE, Wald LL, Setsompop K. Wave-CAIPI for highly accelerated 3D imaging. Magn Reson Med 2015;73:2152-2162.
2. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: Sensitivity encoding for fast MRI. Magn Reson Med 1999;42:952-962.
3. Uecker M, Lai P, Murphy MJ, etc. ESPIRiT—an eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn Reson Med 2014, 71: 990-1001.
4. Lustig M and Pauly JM. SPIRiT: Iterative self‐consistent parallel imaging reconstruction from arbitrary k‐space. Magn Reson Med 2010, 64: 457-471.
5. Griswold MA, Kannengiesser S, Heidemann RM, Wang J and Jakob PM. Field‐of‐view limitations in parallel imaging. Magn Reson Med 2004, 52: 1118-1126.
6. Jia S, Zhang L, Ren L, etc. Joint Intracranial and Carotid Vessel Wall Imaging in 5 minutes using Compressed Sensing accelerated DANTE-SPACE. Eur Radiol 2019, 30:119-127.
7. Wang H, Qiu Z, Su S, Jia S, Li Y, Liu X, Zheng H, Liang D. Parameter optimization framework on wave gradients of Wave-CAIPI imaging. Magn Reson Med 2020;83:1659-1672.
8. Su S, Qiu Z, Luo C, Shi C, Wan L, Zhu Y, Li Y, Liu X, Zheng H, Liang D, Wang H. Accelerated 3D bSSFP Using A Modified Wave-CAIPI Technique with Truncated Wave Gradients. IEEE Transactions on Medical Imaging. 2020. DOI 10.1109/TMI.2020.3021737.
9. Polak D, Cauley S, Huang SY, Longo MG, Conklin J, Bilgic B, Ohringer N, Raithel E, Bachert P, Wald LL, Setsompop K. Highly-accelerated volumetric brain examination using optimized Wave-CAIPI encoding. J Magn Reson Imaging 2019;50:961-974.
10. Qiu Z, Jia S, Su S, Zhu Y, Zheng H, Ying L, Wang H, Liang D. Wave-CAIPI Highly Accelerated Whole-Brain Intracranial Vessel Wall Imaging. Proc. Intl. Soc. Mag. Reson. Med. 2020. p. 2133.
Figures