1153
Calibrationless Parallel Imaging Reconstruction for Simultaneous Multi-slice PROPELLER of Upper Abdomen1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, China, 3Siemens Shenzhen Magnetic Resonance Ltd, Shenzhen, China, 4Center for Biomedical Imaging Research, Department of Biomedical Engineering, Tsinghua University, Beijing, China
Synopsis
This study presents a calibrationless parallel imaging (CPI) reconstruction for simultaneous multi-slice (SMS) PROPELLER MRI of the upper abdomen. With simultaneously excited slices having different blipped-CAIPI shifts, the inherent incoherency of SMS PROPELLER data enables CPI reconstruction via low rank matrix approximation. The proposed method was evaluated with both simulated phantom and acquired abdominal MR data. Compared to conventional split slice-GRAPPA, the proposed method jointly reconstructs all blades, providing significantly improved SNR and reduced artifact level.
Introduction
PROPELLER MRI has shown its effectiveness and efficiency for motion compensation in abdominal imaging1,2, but it prolongs the scan time compared to Cartesian acquisition. In practice, PROPELLER MRI can be accelerated using parallel imaging with in-plane undersampling and/or simultaneous multi-slice (SMS) acquisition. Conventionally, each blade can be recovered with parallel imaging reconstruction, then combined to form an image3,4. However, reconstructing individual blades separately can cause severe noise amplification, especially at high undersampling factors. Moreover, calibration-based methods rely on additionally acquired calibration data, which further prolongs the acquisition time, and can be sensitive to the mismatch between the calibration and PROPELLER data.Besides motion compensation capability, PROPELLER MRI samples the k-space in an incoherent way. Specifically, undersampled blades with different rotation angles spread the aliasing along different directions. In SMS PROPELLER, with simultaneously excited/acquired slices having different blipped-CAIPI shifts, each slice has distinct aliasing spreading patterns, leading to incoherency along slice direction. Such characteristics can enable calibrationless parallel imaging reconstruction and exploitation of coil sensitivity encoding capabilities in both in-plane and slice directions.
In this study, a calibrationless parallel imaging (CPI) reconstruction method was proposed for SMS PROPELLER. It jointly reconstructs all blades, with parallel imaging constraint for each slice promoted via low-rank matrix approximation5,6, and data consistency enforced by minimizing the difference between synthesized and acquired SMS PROPELLER data. The proposed approach was evaluated with both simulation and in vivo studies.
Method
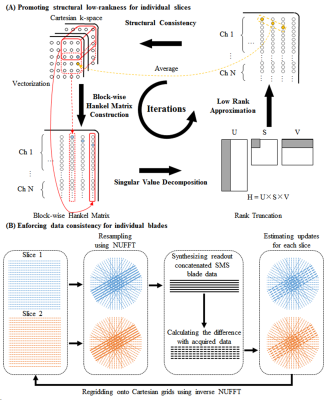
Proposed ReconstructionThe proposed method simultaneously performs parallel imaging reconstruction and blade combination, it iteratively updates the estimated k-space by sequentially promoting structural low-rankness and enforcing data consistency (Figure 1). Specifically, structural low-rankness is promoted for individual slices by constructing a block-wise Hankel matrix, performing singular value decomposition, forcing low-rankness through rank truncation, and structural consistency by averaging the elements corresponding to the same k-space sample5,6. The data consistency is enforced for individual blades by resampling the PROPELLER data for each slice, synthesizing readout concatenated SMS blade data7, calculating the difference with acquired data, estimating the updates for each slice on PROPELLER trajectories, and regridding the updates onto Cartesian grids using inverse NUFFT.
Evaluation with Simulations Study
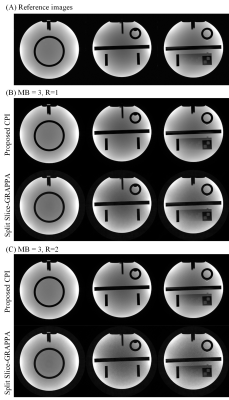
Phantom data were acquired on a Siemens 1.5T MAGNETOM Aera scanner equipped with a 20-channel head coil, using spin echo sequence with TR/TE=550/9ms, matrix size=320×256, FOV=230×230mm2. The acquired k-space data were retrospectively resampled following a PROPELLER trajectory with number of samples along readout=240, blade width=32, blade number=12, and used to synthesize SMS PROPELLER data at MB=3/R=1, and MB=3/R=2, where MB denotes multiband factor and R denotes in-plane acceleration factor. 3 selected slices were simulated to be simultaneously excited/acquired with blipped-CAIPI shift set to i/(MB×R) FOV (i=0,1,2). The proposed method was performed with kernel size=6×6, normalized target rank=1.81. The iteration process stopped when the update of k-space data estimation was lower than 0.1‰. The proposed method was compared to conventional split slice-GRAPPA (SPSG) reconstruction, which reconstructed each blade independently and combined all blades after phase correction. SPSG was performed with the first blade as calibration data, kernel size=5×5, regularization factor=0.0001.
Evaluation with In Vivo Study
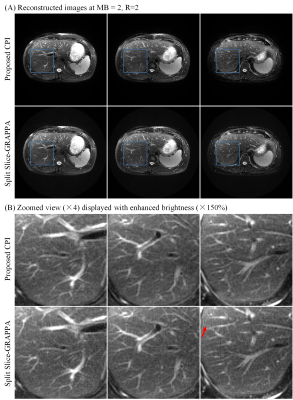
Human abdominal MR data were acquired with a prototype SMS PROPELLER sequence8 on a Siemens 1.5T MAGNETOM Aera scanner using 18-channel body and 24-channel spine coils, with 30 channels adaptively selected. At MB=2/R=2, data were acquired with TR/TE=2000/107ms, echo train length (ETL)=36, blade number=10. At MB=3/R=2, data were acquired with TR/TE=2000/110ms, ETL=30, blade number=12. Other parameters were FOV=380×380mm2, slice thickness/gap=6/1mm, slice number=24, matrix size=320×320, refocusing flip angle=150°. Blipped-CAIPI shift was set to i/(MB×R) FOV (i=0,1,…,MB-1). Calibration data for SPSG was acquired using GRE with matrix size=64×32. The data were acquired using navigator-triggered prospective acquisition correction (PACE) for respiratory motion compensation, and SPAIR (SPectral Attenuated Inversion Recovery) for fat suppression. The proposed reconstruction was performed with kernel size=6×6, normalized target rank=1.17, the iteration process stopped when the update was lower than 1‰.
Results
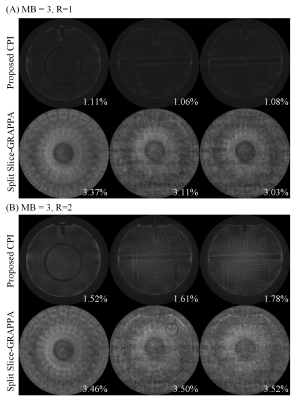
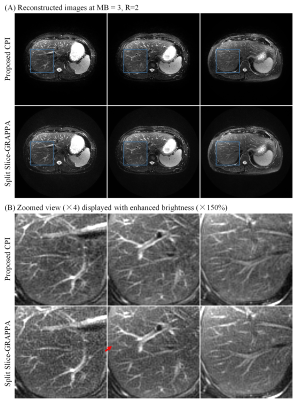
Figure 2 displays the images reconstructed from simulated SMS PROPELLER data at MB=3/R=1, and MB=3/R=2. The SPSG results suffered from severe noise amplification, especially at MB=3/R=2. With CPI reconstruction, the residual error was significantly lower compared with SPSG (Figure 3). Both CPI and SPSG were quantitatively evaluated using root mean square errors (RMSE). CPI had substantially reduced residual error, with ~70% and ~50% reduction of RMSE compared to SPSG at MB=3/R=1, and MB=3/R=2, respectively. Figure 4 shows representative slices for in vivo results at MB=2/R=2, where CPI provided improved results over SPSG in terms of SNR and residual artifacts. Figure 5 shows the images from the same slice locations at MB=3/R=2. At higher multiband factor, such improvement became even more significant.Discussion
The incoherency of SMS PROPELER data can enable robust CPI reconstruction. The proposed CPI reconstruction jointly reconstructs all blades, thus substantially improves the SNR. Note that, with free-breathing, respiratory motion induced corruption of calibration data, and/or dramatic mismatch between calibration and PROPELLER data can undermine the performance of calibration-based parallel imaging reconstruction. Future studies will evaluate the robustness of the proposed CPI reconstruction over conventional SPSG reconstruction in such scenario.Conclusions
The proposed CPI can provide robust reconstruction for SMS PROPELLER of upper abdomen, outperforms conventional SPSG reconstruction in terms of improved SNR and reduced residual artifacts.Acknowledgements
This study was supported by Hong Kong Research Grant Council (R7003-19, C7048-16G, HKU17112120, HKU17103819 and HKU17104020), Guangdong Key Technologies for Treatment of Brain Disorders (2018B030332001), and Guangdong Key Technologies for Alzheimer’s Disease Diagnosis and Treatment (2018B030336001).References
[1] Pipe JG. Motion correction with PROPELLER MRI: application to head motion and free-breathing cardiac imaging. Magn Reson Med 1999;42(5):963-969.
[2] Hirokawa Y, Isoda H, Maetani YS et al. MRI Artifact Reduction and Quality Improvement in the Upper Abdomen with PROPELLER and Prospective Acquisition Correction (PACE) Technique. American Journal of Roentgenology 2008;191(4):1154-1158.
[3] Norbeck O, Avventi E, Engstrom M, Ryden H, Skare S. Simultaneous multi-slice combined with PROPELLER. Magn Reson Med 2018;80(2):496-506.
[4] Chang Y, Pipe JG, Karis JP et al. The effects of SENSE on PROPELLER imaging. Magn Reson Med 2015;74(6):1598-1608.
[5] Shin PJ, Larson PE, Ohliger MA et al. Calibrationless parallel imaging reconstruction based on structured low-rank matrix completion. Magn Reson Med 2014;72(4):959-970.
[6] Liu Y, Yi Z, Zhao Y et al. Calibrationless Parallel Imaging Reconstruction for Multislice MR Data using Low-Rank Tensor Completion. Magn Reson Med 2020;85(2):897-911.
[7] Koopmans PJ. Two-dimensional-NGC-SENSE-GRAPPA for fast, ghosting-robust reconstruction of in-plane and slice-accelerated blipped-CAIPI echo planar imaging. Magn Reson Med 2017;77(3):998-1009.
[8] Zhou K, Liu W, Dong F, Cheng S. Slice-GRAPPA calibration using pre-scan data and application to simultaneous multi-slice PROPELLER. In: 2017 Proceedings of International Society for Magnetic Resonance in Medicine (ISMRM), Honolulu, 2017, p 3842.
Figures