1150
Time-dependent IVIM/Non-Gaussian parameters between in vivo and post-mortem breast cancer xenograft models1Department of Diagnostic Imaging and Nuclear Medicine, Kyoto University, Kyoto, Japan, 2Department of Clinical Innovative medicine, Institute for advancement of clinical and translational science, Kyoto University Hospital, Kyoto, Japan, 3Department of Systems science, Graduate School of Informatics, Kyoto University, Kyoto, Japan, 4NeuroSpin/Joliot, CEA-Saclay Center, Paris-Saclay University, Gif-sur-Yvette, France, 5Human Brain Research Center, Kyoto University Graduate School of Medicine, Kyoto, Japan, 6National Institute for Physiological Sciences, Okazaki, Japan
Synopsis
We investigated the time-dependency of IVIM /non-Gaussian diffusion parameters in vivo and post-mortem using breast xenograft models (MDA-MB-231). fIVIM, perfusion-related fraction, dropped abruptly post-mortem, though not reaching 0 completely, suggesting imperfect modeling. fIVIM, estimated from the pseudo-diffusion model, was independent of the diffusion time. The significant decrease of sADC and ADC0 values and the increase in K values observed between short and long diffusion times, as well as after sacrification are in accordance with an increase in diffusion hindrance, as more water molecules hit many boundaries, such as cell membranes. However, different mechanisms may be at stake.
Introduction
IVIM MRI has the potential to provide quantitative information on microcirculation (perfusion) in various cancers non-invasively, and the increase of fIVIM, perfusion-related fraction, has been reported with the proliferation of neovascularity in some tumors1. Thus, IVIM parameters are expected to play an important role as an imaging modality to reveal the degree of tumor angiogenesis, which is essential for tumor growth and metastasis, without the need for contrast agents. Non-Gaussian diffusion MRI parameters have been also reported to be useful especially in oncologic imaging2, because these parameters are more sensitive to water diffusion hindrance by constitutive tissue elements like cell membranes. However, both IVIM and non-Gaussian diffusion parameters, which are often reported together, may depend on the diffusion time3,4, although this factor is rarely reported. The change of IVIM/ non-Gaussian diffusion parameters with different diffusion times may provide additional information on tumor tissue microstructure, membrane permeability and blood microcirculation patterns. Hence, we investigated 1) the time-dependency of IVIM /non-Gaussian diffusion parameters in vivo and post-mortem conditions; 2) the correlation between IVIM parameters and vascularity by histological evaluation, using breast xenograft models (MDA-MB-231). IVIM scanning was performed in-vivo and post-mortem conditions to obtain the perfusion-driven effects.Materials & Methods
Nine ICR nu/nu mice were injected subcutaneously with 1×106 MDA-MB-231 cells in the hind limb. MRI was conducted on a 7T MRI scanner (Bruker, Germany) using a 1H quadrature transmit/receive volume coil. The SE-EPI acquisition parameters were set as follows; Resolution 250 x 250μm², matrix size 100 x 100, field of view 25 x 25 mm² , slice thickness 1.5 mm, TE=57ms, TR=2500 ms, 8 averages, 4 segments. IVIM/ Diffusion-weighted (DW) images were acquired using 2 different diffusion times (9 and 27.6ms) and 19 b values (7-4105 s/mm²). They were measured both in vivo and post-mortem. The acquisition time was 22 min 40 sec. Shifted ADC values were calculated using b values of 200 and 1500 sec/mm2 5. Non-Gaussian diffusion and fIVIM values were estimated using the combined IVIM/non-Gaussian diffusion kurtosis model6. Xenograft tumors were harvested and stained immunohistochemically for CD31. CD31-positive microvessel density (MVD) was measured. MRI data analysis was performed using a code developed in Matlab (Mathworks, Natick, MA). Diffusion parameters were compared using Wilcoxon test, and the correlation of MR parameters with MVD were investigated using the Spearman's rank correlation test.Results
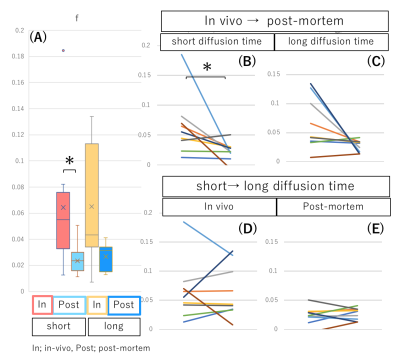
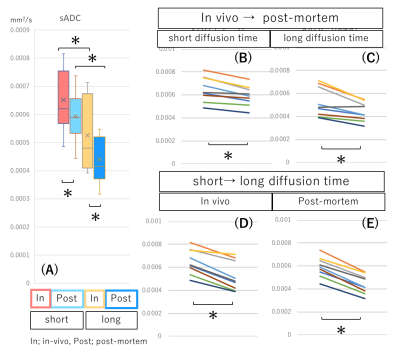
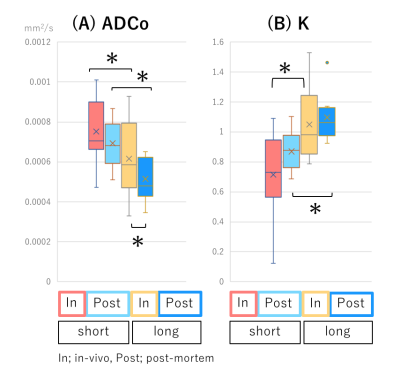
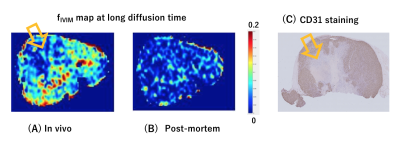
fIVIM values significantly dropped after sacrification at short diffusion time (Mean±SD; 6.4±5.0% vs. 2.4±1.5%; P<0.05) and tended to decrease post-mortem at long diffusion time (6.5±4.5% vs. 2.6±1.0%; P=0.054). Interestingly, fIVIM values post-mortem didn’t reach 0 despite lack of perfusion (Fig.1). sADC values significantly decreased both at longer diffusion times and after sacrification (Fig.2, P<0.05). ADC0 values had almost same result as sADC (Fig.3), however, ADC0 at short diffusion time tended to decrease after sacrification (Fig.3, P=0.054). Post-mortem tumor had larger change as diffusion time increased, compared with in vivo tumor, in both sADC and ADC0 (P<0.05). Time-dependency of K were shown both in vivo and post-mortem (Fig.3, P<0.05). After sacrification, K were showed mild increase (Fig.3, P=0.16 at long diffusion time; P=0.054 at short diffusion time, respectively). A representative case of fIVIM maps was shown in Fig.4. Higher fIVIM area was well correlated with CD31-positive brownish area. There was no significant association between fIVIM and MVD.Discussion
The results clearly show that IVIM reflects perfusion in tissues, as perfusion-related IVIM values dropped abruptly post-mortem as expected, due to the lack of perfusion. However, fIVIM did not reach 0, which might appear surprising. This pitfall underlines the limitation of the Kurtosis model, especially when the diffusion component is not fully taken into account and when noise floor effects are not fully corrected2. Basically, fIVIM was found independent of the diffusion time, however, although some time-dependency could be expected. We used the IVIM exponential model, which is valid as long as diffusion time is long enough, however the sinc model might be more suitable at short time scales2,4. The correlation between fIVIM and MVD was not significant in this study. The association between MVD and IVIM is reported by several studies1, and further investigation is warranted to verify the correlation. The change in ADC values and K obtained from scans after sacrification suggests an increase of hindered/restricted diffusion effects, possibly due to cytotoxic edema linked to ischemic damage and the resulting decrease of extracellular space. Changes in cell membrane permeability may also play a role7. The significant decrease of ADC values and the increase in K values observed with longer diffusion time, which are compatible with the assumption that diffusion hindrance increases with increasing diffusion time, as more water molecules hit many boundaries, such as cell membranes3. With longer diffusion time, the change in ADC values of post-mortem tumor was larger, reflecting more hindered diffusion behavior.Conclusion
fIVIM from IVIM MRI is a promising quantitative parameter to evaluate blood microcirculation in tumors. However, care should be taken to estimate tissue diffusion effects as accurately as possible and correct for noise floor effects. The change in ADC/Non-Gaussian diffusion parameters as diffusion time increased might provide additional insights on tissue microstructure and hindrance mechanisms.Acknowledgements
This work was supported by the Uehara Memorial Foundation for Life Science and AMED grant number under Grant Number JP18ck0106454.References
1) Iima M. Perfusion-driven Intravoxel Incoherent Motion (IVIM) MRI in Oncology: Applications, Challenges, and Future Trends. Magn. Reson. Med. Sci. 2020 Jun 15. doi: 10.2463/mrms.rev.2019-0124.
2) Le Bihan D. What can we see with IVIM MRI? Neuroimage 2019; 187: 56–67.
3) Reynaud O et al. Time-Dependent Diffusion MRI in Cancer: Tissue Modeling and Applications. Frontiers in Physics 2017; 58: 5.
4) Fournet G, Li J-R, Cerjanic AM, et al. A two-pool model to describe the IVIM cerebral perfusion. J. Cereb. Blood Flow Metab. 2017; 37(8): 2987–3000.
5) Iima M, Le Bihan D. Clinical intravoxel incoherent motion and diffusion MR imaging: past, present, and future. Radiology 2016; 278: 13–32.
6) Iima M, Yano K, Kataoka M, et al. Quantitative non- Gaussian diffusion and intravoxel incoherent motion magnetic resonance imaging: differentiation of malignant and benign breast lesions. Invest Radiol 2015; 50: 205–211.
7) Jelescu IO, Ciobanu L, Le Bihan D et al. Effects of hypotonic stress and ouabain on the apparent diffusion coefficient of water at cellular and tissue levels in Aplysia. NMR Biomed. 2014; 27: 280–290.
Figures