1146
Impact of low-rank denoising on abbreviated breast diffusion-weighted acquisitions: accuracy and repeatability1Radiology / Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 2Radiology, Stanford University, Palo Alto, CA, United States, 3Electrical and Computer Engineering /CMRR, University of Minnesota, Minneapolis, MN, United States, 4Radiology, University of Minnesota, Minneapolis, MN, United States
Synopsis
This study explores the use of a low-rank denoising technique (NORDIC) on breast DWI. Accuracy and repeatability were assessed by subdividing full acquisitions into shorter scans, calculating ADC with and without NORDIC, and comparing the resultant ADC maps. We found that the denoising effects were dependent on SNR – low SNR regions (e.g., background) had greater denoising efficacy than high SNR regions. These findings indicate that NORDIC may help improve assessment of small lesions, and could be used to prospectively optimize higher-resolution acquisition protocols.
Introduction
Diffusion weighted imaging (DWI) is increasingly being used in breast MRI scan protocols to help improve diagnostic performance and monitor response to treatment (1). With currently methods, breast DWI has substantial artifacts and low SNR, which limit its clinical utility. Recent work has shown the feasibility of using low-rank enforcing methods to denoise source diffusion images prior to fiber tracking. The MPPCA method of Veraart et al. performs a patch-based singular valued decomposition on series of DWI volumes and zeros out those eigenvalues attributable to asymptotic properties of random noise (2). The NORDIC method of Moeller et al. refines this approach using normalization of the spatially varying thermal noise and using an invariant noise threshold (3).In this work we explore the application of these low-rank denoising methods to conventional breast DWI. While denoising methods have been explored for diffusion tensor imaging of the breast (4), conventional breast DWI is typically acquired with only a few b-values and directions, and thus has fewer repeated volumes to separate thermal noise from true signal with spatial and temporal covariance. We retrospectively simulated ~2x and 4x abbreviated acquisitions of standard DWI breast patient data by reducing the number of averages per b-value. Denoising was applied to the abbreviated series, followed by estimation of the apparent diffusion coefficient (ADC). We quantitatively measured ADC accuracy of these shortened acquisitions relative to the full acquisition and scan-scan repeatability to objectively assess the impact of denoising.
Methods
In this IRB-approved study, 40 women receiving MRI scans for monitoring treatment response to breast cancer consented to additional diffusion scans; of these 17 participants had their raw data saved and available for use in this retrospective study. The diffusion acquisition consisted of an axial single-shot spin-echo echo-planar acquisition (TR/TE = 8000/74 ms, matrix 192 x 192, nominal resolution 1.7 x 1.7mm, echo spacing 0.74 ms, 36-44 slices 4mm thick, GRAPPA R=3), bipolar diffusion encoding with four b-values (0/100/600/800 s/mm2) acquired in three directions, multiple averages per b-value (NEX = 5/9/9/9 respectively) in a total scan time of 4 min 58 s (5).From the full scans, each acquisition was subdivided into two scans with approximately half of the averages per b-value (50% NEX, with 2/4/4/4 averages), and two with one fourth of the averages each (25% NEX, with 1/2/2/2 averages). The full acquisition and each of the abbreviated acquisitions were independently denoised using NORDIC denoising (3) in magnitude mode using noise-flattening and Marchenko-Pastur estimation.
All series were fit with an exponential decay to produce ADC maps. To select the high-SNR regions, ADC maps were masked by the top 10% and 1% of pixels in the b=0 s/mm2 image. Accuracy of abbreviated acquisitions was measured as the mean absolute error between the abbreviated ADC map and the full-acquisition map. Repeatability was assessed by measuring the mean absolute difference between simulated scan-rescan pairs.
Results
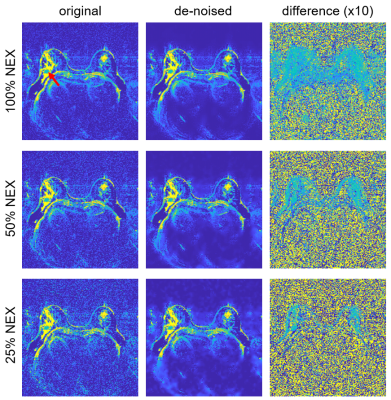
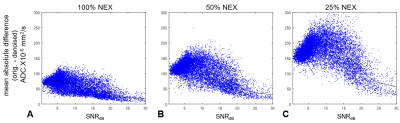
Representative examples of ADC maps calculated from original and denoised data are shown in Figure 1. As evident in the difference images (right), the denoising effect is not spatially uniform: regions with high signal are less affected by the denoising, whereas regions of moderate and low signal show substantial removal of Gaussian noise. Similarly, the more abbreviated acquisitions (with lower signal) show more effective noise reduction across all region in the image. In all cases, no evidence of artifacts or spatial blurring were observed.Figure 2 demonstrates the dependency of the denoising technique on image SNR using a patch-based analysis. While the denoising process affects patches from all SNR regions, the effect is greatest in the low- and mid-SNR range and smallest in the patches with highest SNR.
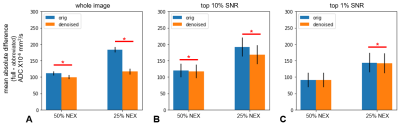
The accuracy of ADC estimates from abbreviated acquisitions is plotted in Figure 3. NORDIC denoising improves the ADC accuracy for both the 50% and 25% NEX acquisitions when assessed over the whole image. The improvement in accuracy is reduced when considering only the high SNR pixels.
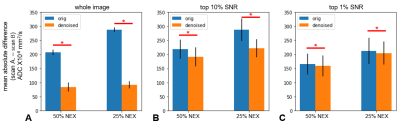
Repeatability between the scan-rescan pairs is shown in Figure 4. While denoising improves repeatability (i.e. reduces differences between ADC estimates) in all comparisons, the effect is largest when SNR is lowest.
Discussion
This study demonstrates the feasibility of using low-rank denoising to improve the performance of abbreviated breast DWI acquisitions without penalty of blurring or artifacts. The impact is consistently measurable in all regions, but has a modest effect in the highest SNR regions such as the tumor in Figure 1. For this acquisition protocol the greatest benefit would likely be in improving visualizations of small lesions.Note that this application employed denoising with far fewer volumes than has been previously used in DWI applications: the 50% NEX scan had 14 volumes, and 25% NEX had 7 volumes, whereas prior work in brain imaging has typically used 90 or more volumes (2,3). Furthermore, processing the complex images rather than magnitude data is likely to provide greater denoising performance.
Conclusion
Retrospective low-rank denoising can improve accuracy and quantitative reproducibility in conventional breast DWI with few volumes, with larger improvements seen in regions with low SNR.Acknowledgements
NIH P41 EB027061, NIH S10OD017974-01, and NIH R21CA201834References
1. Iima M, Honda M, Sigmund EE, Kishimoto AO, Kataoka M, Togashi K. Diffusion MRI of the breast: Current status and future directions. Journal of Magnetic Resonance Imaging 2020;52:70–90 doi: https://doi.org/10.1002/jmri.26908.
2. Veraart J, Novikov DS, Christiaens D, Ades-aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. NeuroImage 2016;142:394–406 doi: 10.1016/j.neuroimage.2016.08.016.
3. Moeller S, Pisharady PK, Ramanna S, et al. NOise reduction with DIstribution Corrected (NORDIC) PCA in dMRI with complex-valued parameter-free locally low-rank processing. NeuroImage 2021;226:117539 doi: 10.1016/j.neuroimage.2020.117539.
4. Tan ET, Wilmes LJ, Joe BN, et al. Denoising and Multiple Tissue Compartment Visualization of Multi‐b‐Valued Breast Diffusion MRI. J Magn Reson Imaging 2021;53:271–282 doi: 10.1002/jmri.27268.
5. McKay JA, Church AL, Rubin N, et al. A Comparison of Methods for High-Spatial-Resolution Diffusion-weighted Imaging in Breast MRI. Radiology 2020:200221 doi: 10.1148/radiol.2020200221.
Figures