1136
Increased Saturated Fatty Acid Fraction in the Adipose Tissue Near Malignant Tumors in Breast Cancer Patients1Radiology, Weill Cornell Medicine, New York, NY, United States, 2Radiology, NYU Langone Health, New York, NY, United States
Synopsis
We assessed the distribution of saturated fatty acid (SFA) in the adipose tissue around malignant tumors and the whole breast of patients with benign and malignant lesions. Bilateral gradient echo spectroscopic imaging sequence was acquired simultaneously. A voxel-wise analysis in the frequency domain was then applied to measure the SFA in each breast's adipose tissues and peri-tumoral region. Our results showed that the SFA was significantly higher around the malignant tumor than on the contralateral side, while no significant changes were observed with benign lesions. The results indicate that the SFA may be closely associated with the malignancy of lesions.
Introduction
The breast is composed mainly of fibroglandular and adipose tissue (1). While breast cancer typically originates from the epithelial cells in breast glandular tissue (2,3), it seems that the tumor initiates and progresses at the boundary with adipose or other surrounding tissues (3). Studies have also discovered cross-talk between breast tumor cells and neighboring adipocytes (3,4). These findings suggest that adipocytes play an active part in the development of breast cancer. In addition to the overall amount of body fat, fatty acid composition (FAC) may also be important in cancer development. The purpose of the study is to compare the saturated fatty acid in the mammary adipose tissues around malignant and benign lesions with those in the ipsi- and contralateral breasts.Methods
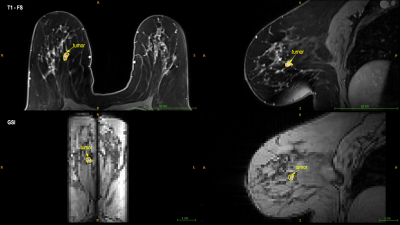
Nine women with a malignant and/or benign (age: 63 ± 7 years) lesion in one breast were recruited for this IRB-approved study. Bilateral scans were acquired using the 3D gradient-echo spectroscopic imaging (GSI) sequence with dual slab excitation (5). on a whole-body 3T MRI scanner (TIM Trio; Siemens, Erlangen, Germany) with a dedicated 16-element bilateral breast coil (In vivo, Orlando, FL). The imaging parameters were: 2.8mm × 2.8mm × 2.8mm, GRAPPA acceleration factor = 8, inter-echo spacing = 1.44ms, receiver bandwidth = 1410 Hz/pixel, flip angle =10° and TR = 213ms. The total scan time was 5:27 minutes. A clinical fat-saturated T1-weighted scan was also acquired, and the ROIs were drawn in the clinical image by a fellowship-trained breast imaging radiologist with four years of experience. The ROIs were then mapped on the GSI images for further analysis. Fatty acid composition analysis was performed to measure the saturated fatty acid (SFA) in the adipose tissue for both ipsi- and contralateral breast as well as small ROI around the tumor.Results
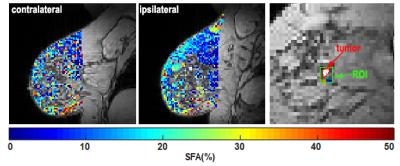
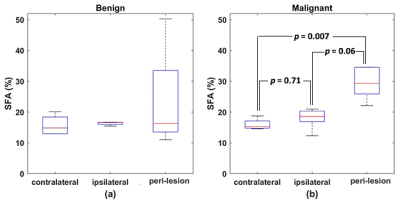
Figure 1 shows an example of ROI drawn on clinical and GSI images. An example of SFA maps in both breasts and around the tumor in patients with malignant and benign lesions are shown in Figure 2. Figure 3 shows the boxplot comparison of SFA between the whole contra and ipsilateral breast and the peri-lesion ROI for benign and malignant lesions. Although the SFA is higher in the ipsilateral breast than the contralateral breast in patients with the malignant lesion, their difference is not significant (p=0.71). In contrast, a significantly higher (p=0.007) SFA was observed in the adipose tissue around the tumor compared to those in the ipsi and contralateral breasts. No significant changes were observed for benign lesions.Discussion and Conclusion
The higher SFA measurement around the tumor observed in this study agreed with previous mammary adipose tissue studies (6). The voxel-wise spectral analysis used in this study can effectively account for spatial variation in B0 and T2* differences among lipid peaks. The results of this proof-of-concept study demonstrate the importance of the spatial distribution of fatty acid composition with respect to the lesion location. We plan to extend to a larger cohort of women with malignant and benign lesions to assess the FAC's diagnostic utility further.Acknowledgements
This work was supported by grants R01CA160620, R01CA219964, and UG3CA228699 from the National Institutes of Health.References
1. Ekpo EU, Hogg P, Highnam R, McEntee MF. Breast composition: Measurement and clinical use. Radiography 2015;21(4):324-333.
2. Elenbaas B, Spirio L, Koerner F, Fleming MD, Zimonjic DB, Donaher JL, Popescu NC, Hahn WC, Weinberg RA. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes and Development 2001;15(1):50-65.
3. Zhu W, Harvey S, Macura KJ, Euhus DM, Artemov D. Invasive Breast Cancer Preferably and Predominantly Occurs at the Interface Between Fibroglandular and Adipose Tissue. Clinical Breast Cancer 2017;17(1):e11-e18.
4. Andarawewa KL, Motrescu ER, Chenard MP, Gansmuller A, Stoll I, Tomasetto C, Rio MC. Stromelysin-3 is a potent negative regulator of adipogenesis participating to cancer cell-adipocyte interaction/crosstalk at the tumor invasive front. Cancer Research 2005;65(23):10862-10871.
5. Storey P, Moy LM, Kim SG. A dual-slab 3D gradient-echo spectroscopic imaging sequence with correction of respiration- and hardware-related frequency variations for bilateral evaluation of lipid composition in the breast. ISMRM 2019; Montreal. p 1872.
6. Freed M, Storey P, Lewin AA, Babb J, Moccaldi M, Moy L, Kim SG. Evaluation of Breast Lipid Composition in Patients with Benign Tissue and Cancer by Using Multiple Gradient-Echo MR Imaging. Radiology 2016;281(1):43-53.
Figures