1135
Feasibility of respiratory self-gated free breathing supine breast DCE-MRI using data-driven model consistency condition reconstruction
Ping N Wang1, Julia V Velikina2, Alexey A Samsonov2, Lloyd Estkowski3, Ty A Cashen3, Frederick Felcz2, Roberta M Strigel1,2,4, Frank R Korosec1,2, and James H Holmes2
1Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Global MR Applications & Workflow, GE Healthcare, Madison, WI, United States, 4Carbone Cancer Center, University of Wisconsin-Madison, Madison, WI, United States
1Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Global MR Applications & Workflow, GE Healthcare, Madison, WI, United States, 4Carbone Cancer Center, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Advanced acquisition and reconstruction methods have been proposed to improve spatial and temporal resolution for prone breast DCE. However, limited work has been performed in the supine setting, which is used in most downstream clinical services including surgery and biopsy. In this work, we propose a radial acquisition combined with self-gating and MOCCO reconstruction. We assess performance of these methods using a simulated breast phantom with respiratory motion and contrast kinetics allowing evaluation of reconstruction accuracy against the assigned ground truth. Evaluation of both spatial and temporal quality was also performed in a first in-vivo patient volunteer.
Introduction
Breast MRI is conventionally performed in the prone position using breast specific RF coils. This has the advantages of minimizing respiratory motion during longer time-resolved imaging studies typically needed to acquire sufficient contrast kinetics and spatial resolution with full organ coverage. However, one significant drawback is that this positioning results in significant spatial deformation from that used in of both surgery1,2 and biopsy3,4. Recently, a data-driven compressed sensing method with temporal regularization was used for undersampling radial data to generate high spatial-temporal resolution images in prone breast DCE-MRI5. At the same time, significant progress has been made in liver DCE to develop time-resolved free-breathing approaches using radial acquisition with advanced reconstruction where effective temporal resolution can be traded off for greater robustness to motion6,7. We hypothesize that these self-navigation techniques can be extended to breast imaging, where their combination with a novel radial stack-of-stars acquisition and data-driven low-rank compressed sensing reconstruction strategy can maintain high temporal resolution.Methods
Reconstruction: Self-gating was performed to group similar respiratory positions and minimize intra-time-frame motion, which was extracted from the DC components of projections along kz. A low pass filter with frequency of 0.6 Hz was applied to the DC series to select respiratory-only signal. A 1D Fourier transform was performed to generate projection intensity profiles from each coil, followed by principle component analysis (PCA). The PC with the highest peak was selected to represent the self-gating respiratory signal. Following this, the four respiratory states were selected and time-resolved images with 15 s temporal resolution were reconstructed using a MOCCO algorithm8 with quadratic (l2) regularization. MOCCO relies on a temporal model of order K (number of temporal basis functions) which is typically estimated from low-resolution images reconstructed from the fully sampled k-space center via principal or independent component analysis (PCA/ICA)9. As the number of projections per image may be low due to rejection of data due to motion, a regularized SENSE10 reconstruction was used to estimate low-resolution images to improve SNR. Model orders K=5 and K=3 were selected for MOCCO reconstruction in simulation and in vivo data, respectively. Iterative SENSE, and compressed sensing with temporal total variation (CS-TV) were also applied for comparison.Simulation: A 3D stack-of-stars radial acquisition was simulated by using a supine breast digital reference object (DRO) that included respiratory motion. Simulated enhancing lesions with higher intensity than the surrounding fat tissue were placed within a region near the axillae (Fig 1, C-F). Different pharmacokinetic parameters (Ktrans, Ve and Vp) based on the extended Tofts model11,12 were assigned to each lesion to model contrast kinetics. A total of 1024 respiratory phases based on the respiratory self-gating signal with frequency of 20 cycles per minute were simulated and the corresponding k-space radial lines were sampled from the motion images. Data from the end-expiration phase (phase 4) were selected using the self-navigation algorithm (Fig 1A) for the contrast enhanced reconstruction.
In-vivo: Imaging was performed using a clinical 3T MR scanner (SIGNA Premier, GE Healthcare, Waukesha, WI) with a lightweight peripheral receive array (AIR Coil, GE Healthcare) and the in-table receive array. A radial stack-of-stars acquisition with golden angle view ordering was performed during a free-breathing contrast injection (Multihance, Bayer Healthcare Pharmaceuticals, Wayne, NJ) followed by a Cartesian acquisition using navigator-gating. Scan parameters included: TR/TE = 4.57/2.08 ms; FOV = 40 cm; flip angle = 15; receiver bandwidth = +/-83.3 kHz; acquisition matrix = 448x448x100; acquisition resolution = 0.9x0.9 mm2 in-plane and 1.4 mm out-of-plane resolution.
Results
Simulations: MOCCO reconstruction with higher model order K=5 demonstrated overall better temporal fidelity than CS-TV across different lesion kinetics (Fig 2). CS-TV showed over-smoothing of lesion curves, especially in rapidly changing lesions (Fig 2, B and H). Both CS-TV and MOCCO showed significant improvement in recovering spatial resolution compared with SENSE (Fig 3). Some residual low-intensity streaking artifact is visible at the edge of the axillae region due to data inconsistency from the chest wall motion.In-vivo: Images reconstructed from MOCCO with model order K=3 and CS-TV allow visualization of enhancing lesions in the fibroglandular tissue without evidence of motion and/or significant undersampling artifacts (Fig 4.). To fully recover the spatial resolution, a high regularization parameter was used for CS-TV resulting in over-constraining of the lesion intensity (Fig 5). Temporal curves measured from MOCCO with K=3 showed better recovery of the temporal fidelity in all three lesion types.
Discussion and conclusions
In this study, we demonstrate the feasibility of providing motion-free high spatial and temporal resolution supine breast DCE-MRI using radial acquisition with MOCCO reconstruction. Both simulation and in vivo data demonstrated that the effective temporal resolution of 15 s with spatial resolution of 0.9x0.9 mm2 was achievable in the setting of supine breast DCE-MRI. Previous simulations without respiratory motion showed the ability to achieve 5 s temporal resolution and 0.8x0.8 mm2 spatial resolution5 such that there is some trade-off for the motion robustness provided by the self-gating. The results also indicated that it may be especially challenging to obtained sufficient temporal basis functions in situations with highly undersampled images due to motion rejection. Future work will investigate obtaining temporal basis functions from other techniques (e.g. dictionary learning13).Acknowledgements
The authors wish to acknowledge support from the following NIH grants: R21EB018483, R01EB027087, P30CA014520, and R01CA248192. As well as support from GE Healthcare, the RSNA Research and Education Foundation, and a Research and Development Grant from the Departments of Radiology and Medical Physics, University of Wisconsin-Madison.References
- Conley RH, Meszoely IM, Weis JA, Pheiffer TS, Arlinghaus LR, Yankeelov TE, Miga MI. Realization of a biomechanical model-assisted image guidance system for breast cancer surgery using supine MRI. International Journal of Computer Assisted Radiology and Surgery. 2015;10(12):1985–1996. doi:10.1007/s11548-015-1235-9
- Perkins SL, Lin MA, Wheeler AJ, Hargreaves BA, Bruce Daniel L. Perceptual Accuracy of a Mixed-Reality System for MR-Guided Breast Surgical Planning in the Operating Room. In: Proceedings of Joint Annual Meeting ISMRM-ESMRMB. Paris, France; 2018. p. 607.
- Telegrafo M, Rella L, Stabile Ianora AA, Angelelli G, Moschetta M. Supine breast US: how to correlate breast lesions from prone MRI. The British Journal of Radiology. 2015;89(1059):20150497. doi:10.1259/bjr.20150497
- Arıbal E, Buğdaycı O. Supplementary abbreviated supine breast MRI following a standard prone breast MRI with single contrast administration: is it effective in detecting the initial contrast-enhancing lesions? Diagnostic and Interventional Radiology. 2019;25(4):265–269. doi:10.5152/dir.2019.18167
- Wang PN, Velikina JV, Strigel RM, Henze Bancroft LC, Samsonov AA, Cashen TA, Wang K, Kelcz F, Johnson KM, Korosec FR, Ersoz A, Holmes JH. Comparison of data-driven and general temporal constraints on compressed sensing for breast DCE MRI. Magn Reson Med. 2020 Dec 11. doi: 10.1002/mrm.28628. Epub ahead of print. PMID: 33306217.
- Weiss J, Taron J, Othman AE, Grimm R, Kuendel M, Martirosian P, Ruff C, Schraml C, Nikolaou K, Notohamiprodjo M. Feasibility of self-gated isotropic radial late-phase MR imaging of the liver. European Radiology. 2017;27(3):985–994. doi:10.1007/s00330-016-4433-0
- Feng L, Axel L, Chandarana H, Block KT, Sodickson DK, Otazo R. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magnetic Resonance in Medicine. 2016;75(2):775–788. doi:https://doi.org/10.1002/mrm.25665
- Velikina JV, Samsonov AA. Reconstruction of dynamic image series from undersampled MRI data using data-driven model consistency condition (MOCCO). Magnetic Resonance in Medicine. 2015;74(5):1279–1290. doi:10.1002/mrm.25513
- Novey M, Adali T. On Extending the Complex FastICA Algorithm to Noncircular Sources. IEEE Transactions on Signal Processing. 2008;56(5):2148–2154. doi:10.1109/TSP.2007.911278
- Liu B, King K, Steckner M, Xie J, Sheng J, Ying L. Regularized sensitivity encoding (SENSE) reconstruction using bregman iterations. Magnetic Resonance in Medicine. 2009;61(1):145–152. doi:https://doi.org/10.1002/mrm.21799
- Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, Larsson HBW, Lee T-Y, Mayr NA, Parker GJM, et al. Estimating kinetic parameters from dynamic contrast-enhanced t1-weighted MRI of a diffusable tracer: Standardized quantities and symbols. Journal of Magnetic Resonance Imaging. 1999;10(3):223–232. doi:10.1002/(SICI)1522-2586(199909)10:3<223::AID-JMRI2>3.0.CO;2-S
- Sourbron SP, Buckley DL. On the scope and interpretation of the Tofts models for DCE-MRI. Magnetic Resonance in Medicine. 2011;66(3):735–745. doi:10.1002/mrm.22861
- Aharon M, Elad M, Bruckstein A. K-SVD: An algorithm for designing overcomplete dictionaries for sparse representation. IEEE Transactions on Signal Processing. 2006;54(11):4311–4322. doi:10.1109/TSP.2006.881199
Figures
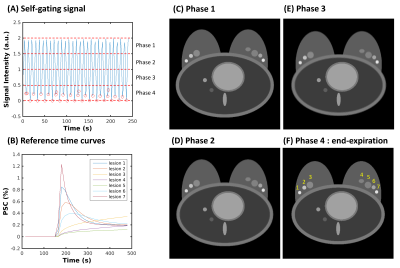
Figure
1: A breast DRO with respiratory motion and simulated lesions generated with
seven different contrast enhancement patterns. Examples of four different
respiratory phases (C-F) generated by averaging the motion images from the
corresponding time points sorted by the respiratory self-gating signal (A). The
accepted radial spokes from end-expiration phase (F) are shown as red circles,
and the corresponding curves of the true percent signal change (PCS) for seven
lesions are shown in (B).
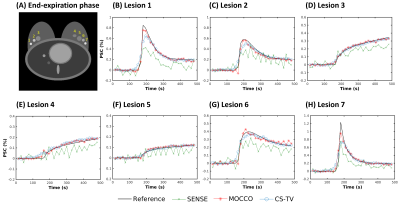
Figure
2: Percent signal change (PSC) measured in ROIs placed in seven lesions with
different contrast kinetics (A). Curves from SENSE (green, X) showed large
oscillations due to the presence of undersampling artifacts. Both MOCCO (red,
stars) and CS-TV (blue, circles) displayed accurate recovery of most lesion
kinetics (C-G). Underestimation of the intensities in lesion 1 (B) and lesion 7
(H) was observed due to the sharp wash-in and wash-out slopes.
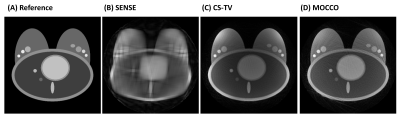
Figure
3: A representative time frame with peak intensity (time = 200 s) comparing
reference image (A) with undersampled reconstructions using SENSE (B), CS-TV
(C), and MOCCO (D) at 15 s temporal resolution. With extreme acceleration R=88,
the spatial resolution can still be recovered by CS-TV and MOCCO, although
residual streaking artifacts are visible in the breast and chest wall. Despite
showing slightly more streaking artifacts, MOCCO provided better temporal
fidelity and sharper lesion edges.
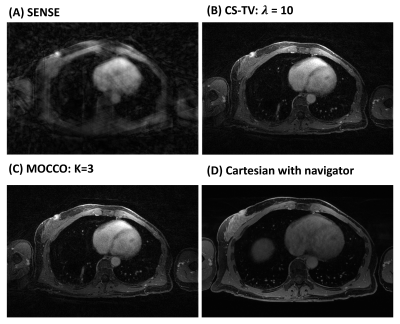
Figure
4: Individual time frames at peak contrast enhancement (time = 240 s)
reconstructed using SENSE (A), CS-TV (B), MOCCO with model order K=3 (C) with
temporal resolution of 15 s. High image quality is observed for both
regularized reconstructions (B, C). Note the late-phase image (D) was acquired
with a navigator-gated Cartesian acquisition, which resulted in a longer acquisition
time (8:43 min:s).
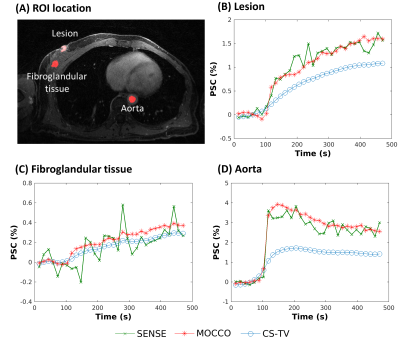
Figure
5: Supine image (A) from a patient volunteer reconstructed using SENSE (green,
X), CS-TV (blue, circles), and MOCCO with model order K=3 (red, stars) with 15
s temporal resolution. PSC (%) are plotted from ROIs placed in the lesion,
fibroglandular tissue, and aorta (A, red outlines). Rapid wash-in and wash-out
contrast kinetics are observed in the aorta D). The lesion B) showed relatively
rapid contrast update, while the fibroglandular tissue C) showed slower
contrast uptake.