1133
Histogram analysis of synthetic MRI parameters correlations with prognostic factors and molecular subtypes in invasive ductal breast cancer1Fudan University Shanghai Cancer Center, Shanghai, China, 2GE Healthcare, Beijing, China
Synopsis
This study quantitatively evaluated intra-tumoral heterogeneity through histogram analysis of synthetic MRI parameters, and determined correlations with prognostic factors and molecular subtypes of breast IDC. We found that IDC with high nuclear grade had higher PD10th, PDmean, PDmedian and PDmax. PDmedian was higher in IDC with HER2 positivity. T110th was higher in cancers with PR negativity. Cancers with hormone receptornegativity had higher T210th, T2mean and T2median than that with hormone receptor positivity. Overall, we demonstrated that whole-tumor histogram-based imaging features derived from synthetic MRI correlated with prognostic factors and may be considered a non-invasive approach for discriminating IDC subtypes.
Materials and methods In total, 122 female patients with primary breast IDC were enrolled in the study. Breast MRI was performed on a 3.0T scanner (SIGNA Pioneer, GE Healthcare, Milwaukee, WI). Routine T1WI, T2WI and DWI were first obtained, followed by synthetic MRI and dynamic contrast-enhanced (DCE)-MRI acquisitions. The detailed acquisition parameters were as follows: (1) synthetic MRI: TR/TE1/TE2 = 4243/17/93 ms, FOV = 360 × 360 mm, matrix = 256 × 256, slice thickness = 5.5 mm; (2) DCE-MRI: TR/TE = 7.2/2.4 ms, flip angle = 10, FOV = 350 × 329 mm. Five post-contrast scans were acquired after intravenous injection of a contrast agent at 2 ml/s with a dose of 0.2 mmol/kg. Data of synthetic MRI was processed using SyMRI 8.0 (SyntheticMR, Linköping, Sweden) to generate quantitative parametric maps (T1, T2, and PD). We first performed a rigid registration between images of CE-MRI and processed MAGIC images. Then ROI of the whole tumor was manually delineated on registered CE-MRI images using ITK-SNAP (http://www.itksnap.org/pmwiki/pmwiki.php). Finally, the ROI was applied to extract histogram features, including mean, minimum, maximum, 10th and 90th percentiles, kurtosis, and skewness, using PyRadiomics [14][pywu2] . Histopathologic results were analyzed by two pathologists. Molecular subtypes were defined based on the St. Gallen consensus, as follows: luminal A (positive for ER and/or PR, with Ki-67<14%); luminal B (positive for ER and/or PR, with overexpressed HER2 or Ki-67≥14%); HER2 (negative for ER and PR, with overexpressed HER2); TN (negative for ER, PR, and HER2).Statistical analyses were performed using SPSS 19.0 (IBM, Armonk, NY). The histogram characteristics of quantitative parameters were compared between types in different prognostic factors using Mann-Whitney U test and among molecular subtypes (luminal A, luminal B, and TN) using Kruskal-Wallis test, followed by the post-hoc test. P value < 0.05/7 = 0.0071 (control for multiple comparisons across seven prognostic factors) for the comparison of prognostic factors and P value < 0.05 for the comparison of subtypes, were considered statistically significant.
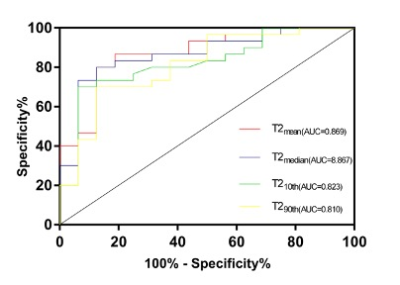
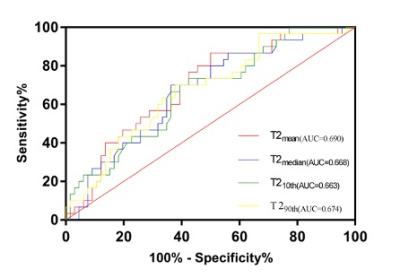
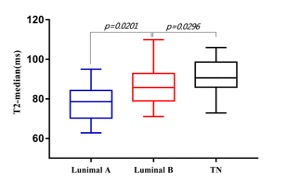
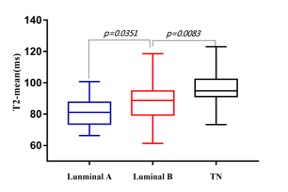
Results For comparisons of histogram characteristics between types in different prognostic factors, we found that PDmedian was significantly higher in cancers with HER2 positivity than that with HER2 negativity. Cancers with high nuclear grade had significantly higher PD10th, PDmean, PDmedian, PDmax and PDkurtosis. The T110th was significantly higher in cancers with PR negativity than that with PR positivity. Cancers with hormone receptor negativity had significantly higher T210th, T2mean and T2median than that with hormone receptor positivity. Cancers with high nuclear grade had significantly higher T210th, T2mean, T2median and T2max. T210th, T2mean, T2median and T290th were found significantly different among tumor subtypes. The post-hoc test revealed that T210th, T2mean, T2median and T290th in TN were significantly higher than luminal A subtypes. Figure 1 shows the ROC results of T2 histogram characteristics in distinguishing TN from luminal A subtypes. T210th, T2mean, T2median and T290th in TN were significantly higher than luminal B subtypes. Figure 2 shows the ROC results of T2 histogram characteristics in distinguishing TN from luminal B subtypes. T2meanand the T2median values of TN, luminal B and luminal A types were arranged in descending order (Figure 3).No significant differences were found in PD and T1 values among tumor subtypes.
Discussion Breast cancer heterogeneity influences poor prognosis thorough therapy resistance. Here we performed a histogram analysis of T1, T2, and PD quantitative parameters obtained from synthetic MRI. We specifically investigated associations of the extracted histogram characteristics with several prognostic factors and molecular subtypes of invasive ductal breast cancers. Overall, we demonstrated that whole-tumor histogram-based imaging features derived from synthetic MRI correlated with prognostic factors and may be considered a non-invasive approach for discriminating IDC subtypes.
Acknowledgements
No acknowledgement found.References
1. Martelotto, L.G., et al., Breast cancer intra-tumor heterogeneity. Breast Cancer Res, 2014. 16(3): p. 210.
2. Huber, K.E., L.A. Carey, and D.E. Wazer, Breast cancer molecular subtypes in patients with locally advanced disease: impact on prognosis, patterns of recurrence, and response to therapy. Semin Radiat Oncol, 2009. 19(4): p. 204-10.
3. Momenimovahed, Z. and H. Salehiniya, Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer (Dove Med Press), 2019. 11: p. 151-164.
4. Goldhirsch, A., et al., Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol, 2005. 16(10): p. 1569-83.
5. Warntjes, J.B., O. Dahlqvist, and P. Lundberg, Novel method for rapid, simultaneous T1, T2*, and proton density quantification. Magn Reson Med, 2007. 57(3): p. 528-37.
6. Granberg, T., et al., Clinical Feasibility of Synthetic MRI in Multiple Sclerosis: A Diagnostic and Volumetric Validation Study. AJNR Am J Neuroradiol, 2016. 37(6): p. 1023-9.
7. Hagiwara, A., et al., Synthetic MRI in the Detection of Multiple Sclerosis Plaques. AJNR Am J Neuroradiol, 2017. 38(2): p. 257-263.
8. Krauss, W., et al., Conventional and synthetic MRI in multiple sclerosis: a comparative study. Eur Radiol, 2018. 28(4): p. 1692-1700.
9. Zhang, W., et al., Synthetic MRI of the lumbar spine at 3.0 T: feasibility and image quality comparison with conventional MRI. Acta Radiol, 2020. 61(4): p. 461-470.
10. Jiang, Y., et al., Quantitative synthetic MRI for evaluation of the lumbar intervertebral disk degeneration in patients with chronic low back pain. Eur J Radiol, 2020. 124: p. 108858.
11. Cui, Y., et al., Diagnosis and Grading of Prostate Cancer by Relaxation Maps From Synthetic MRI. J Magn Reson Imaging, 2020. 52(2): p. 552-564.
12. Matsuda, M., et al., Utility of synthetic MRI in predicting the Ki-67 status of oestrogen receptor-positive breast cancer: a feasibility study.Clin Radiol, 2020. 75(5): p. 398 e1-398 e8.
13. Jung, Y., et al., The feasibility of synthetic MRI in breast cancer patients: comparison of T2 relaxation time with multiecho spin echo T2 mapping method. Br J Radiol, 2018: p. 20180479.
14. van Griethuysen, J. J. M., Fedorov, A., Parmar, C., Hosny, A., Aucoin, N., Narayan, V., Beets-Tan, R. G. H., Fillon-Robin, J. C., Pieper, S., Aerts, H. J. W. L. (2017). Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Research, 77(21), e104–e107
Figures