1128
Evaluation of Lactating Breasts Using Diffusion Tensor Magnetic Resonance Imaging: A Feasibility Study.1Medical Imaging, University of Toronto, Toronto, ON, Canada, 2DDE MRI Solutions Ltd., Tel Aviv, Israel, 3Weizmann Institute of Science, Rehovot, Israel, 4Princess Margaret Cancer Center, Toronto, ON, Canada, 5University of Toronto, Toronto, ON, Canada
Synopsis
The performance of breast DTI in the diagnosis of breast cancer in high-risk lactating patients as compared to DCE imaging was prospectively assessed. The study included 38 patients of which six were diagnosed with breast cancer. All six cancers were detected by DTI and showed significantly lower diffusion coefficients and lower anisotropy in comparison to contralateral normal breast tissue. Sensitivity, specificity and accuracy were respectively, 100%, 93.2%, 93.8% for DTI and 66.7%, 83.8% and 82.5% for DCE. The results showed the potential of breast DTI as an aid in the diagnostic workup of high-risk women during the lactation period.
Introduction:
High-risk women at the stage of lactating are at risk of delayed breast cancer diagnosis given the masking effect of increased mammographic breast density and marked background parenchymal enhancement of contrast-enhanced MRI. Screening during lactation is therefore not contemplated by breast screening programs (1-2). The application of breast diffusion tensor imaging (DTI) in lactating patients was initially characterized in studies of the normal lactating breast tissue (3-4). More recently, DTI performance was investigated in patients with breast cancer (5). These studies demonstrated that the DTI parameters, three directional diffusion coefficients (DDCs), mean diffusivity and maximal anisotropy, were significantly lower in breast cancers of lactating patients in comparison to normal lactating breast tissue, as was previously found for non lactating patients as well (6).Purpose:
To evaluate the performance of breast DTI in the diagnosis of breast cancer in high-risk lactating patients as compared to dynamic contrast enhanced imaging.Study type:
Prospective.Methods:
Between June 2015 and Oct 2020, 38 high risk females underwent 40 breast MRI (median age: 37 years, range: 28-44 years) during their lactation period. Three of the 38 females had a known core biopsy diagnosed unilateral breast cancer, at the time of breast MRI. One patient that was scanned by MRI following a 1st cycle of Chemotherapy was excluded from the quantitative analysis. One patient had consecutive pregnancies and sequential 3 breast MRI tests. Images were acquired on a 3T scanner (Skyra-Fit, Siemens Healthineers) with a 16-channels breast coil (Sentinelle, Invivo). The MRI protocol included axial T1 and T2-weighted images without and with fat suppression, DTI with a spin-echo EPI sequence TE/TR= 86ms/12600ms, b-values 0/700 s/mm2, 30 diffusion-gradient directions, resolution of 1.875x1.875x2.4 mm, and a scan duration of ~ 6-8 min, and a clinical dynamic contrast-enhanced (DCE) protocol with 1 pre and 5 post i.v. Gadobutrol administration (0.1 mmol/kg), each acquisition ~60 seconds with TE/TR/flip angle = 1.72ms/3.86mss/18° and 0.72x0.72x1.2mm or 1.1x0.8x1.1 mm3 resolution. DTI datasets were analyzed using a dedicated software (DDE MRI Solutions, Tel Aviv, Israel) described earlier (6), yielding three eigenvectors and their corresponding eigenvalues, λ1, λ2, λ3, MD, as well as the anisotropy indices: maximal anisotropy, λ1-λ3 and fractional anisotropy (FA). A trained breast radiologist (29 years) blinded to DCE images reviewed the generated DTI parametric color maps and the clinical information.Regions of interest (ROIs) were marked on the identified lesions on the λ1 maps and on (contralateral) healthy breast tissue, yielding mean values for the DTI parameters within the ROIs. Measures of diagnostic accuracy (true/false positive/negative) to discriminate all breasts with and without breast cancer were assessed. Two-tailed Student's t-test was applied for evaluate differences between DTI parameters of breast cancer vs. healthy glandular tissue. Lesions’ size were measured by the longest diameter on λ1 maps
Results and Discussion:
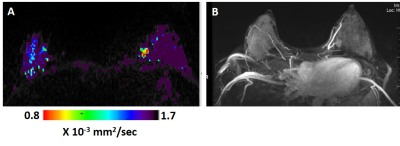
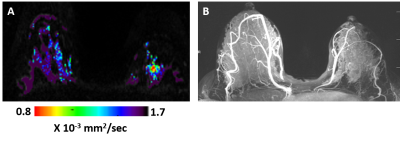
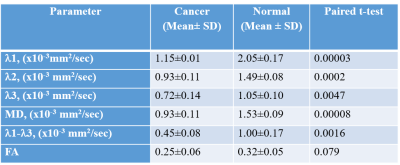
Six invasive ductal carcinomas were diagnosed in 38 patients. All six cancers were detected by DTI (Figures 1 and 2 show examples of two cases) with median cancer size measured by DTI of 15.7 mm (range: 11.1-69.8 mm). Of the 6 cancers, 2 were not detected by DCE, one in a patient with mantle radiation and the other in a BRCA1 carrier (Figure 2). Sensitivity, Specificity and Accuracy were respectively, 100%, 93.2%, 93.8% for DTI and 66.7%, 83.8% and 82.5% for DCE. The DDCs λ1, λ2, λ3, MD and the maximal anisotropy indices λ1-λ3 were significantly lower in the cancer lesions versus their values in the contralateral normal tissue (Table), and their values were similar to previously reported DDCs (3-5) that were acquired with a standardized protocol and analyzed with the same software. The fractional anisotropy in the cancer lesions was not statistically different from it’s value in the normal breasts parenchyma. This finding is in agreement with previous studies that showed that the normalized anisotropy indices fail to differentiate cancer from normal breast tissue (6,7) . Thus far, a median 2.4 years (ranging from 7.5 months to 5 years) follow up included 20 of the patients and indicated that normal lactating breast by DTI parametric λ1 maps did not show false negative results. Further follow up testing are underway.Conclusion:
The above pilot study showed the potential of breast DTI, performed during the lactation period of high-risk patients, to differentiate with high accuracy malignant from normal findings. DTI is a multi-parametric, safe method with intrinsic contrast that directly relates to changes in tissue microstructural features, yet further studies with larger population cohorts are required in order to establish its role in the detection of pregnancy associated breast cancer.Acknowledgements
Dr. E. Furman‐Haran holds the Calin and Elaine Rovinescu Research Fellow Chair for Brain Research. We acknowledge the professional support of Professor Mordechai Sokolov. Dr Scaranelo's research partner, Dr Pavel Crystal ע״ה , was instrumental in defining the path of this research. For this, Dr Scaranelo is extremely grateful to the Joint Department of Medical Imaging (JDMI) in Toronto, Canada.References
1. Ayyappan A.P., et al. Pregnancy-associated breast cancer: spectrum of imaging appearances. Br J Radiol. 83(990):529–34 (2010)
2. Arasu V.A., et al. Imaging the Breast in Pregnant or Lactating Women. Curr Radiol Rep. 6, 10 (2018). 3. Nissan N., et a.l Diffusion-tensor MR imaging of the breast: hormonal regulation. Radiology. 271:672–80 (2014);
4. Nissan N., et al. Monitoring in-vivo the mammary gland microstructure during morphogenesis from lactation to post-weaning using diffusion tensor imaging. J. Mammary Gland Biol. and Neoplasia 22: 193-202 (2017).
5. Nissan N., et al. Breast MRI during lactation: effects on tumor conspicuity using dynamic contrast-enahnced (DCE) in comparison with diffusion tensor imaging (DTI) parametric maps. European Radiology 30:767-777 (2020).
6. Eyal E., et al. Parametric diffusion tensor imaging of the breast. Invest Radiol. 47(5):284-291 (2012)
7. Furman-Haran E., et al. Can diffusion tensor anisotropy indices assist in breast cancer detection ? J. Magn. Reson. Imaging 44(6):1624-1632 (2016).
Figures