1118
A meta-analysis of the diagnostic performance of machine learning–based MRI for axillary lymph node metastasis in breast cancer patients
Chen Chen1, Fabao Gao1, and Xiaoyue Zhou2
1Department of Radiology, West China Hospital, Chengdu, China, 2MR Collaboration, Siemens Healthineers Ltd., Shanghai, China
1Department of Radiology, West China Hospital, Chengdu, China, 2MR Collaboration, Siemens Healthineers Ltd., Shanghai, China
Synopsis
Axillary lymph node dissection (ALND) is the gold standard for evaluating axillary lymph node metastasis (ALNM), but ALND may not confer a survival advantage. Therefore, reliable, noninvasive approaches for preoperative prediction of ALNM have been needed. The use of machine learning (ML) in predicting ALNM in breast cancer patients has been reported. We have conducted a large-sample-size assessment and a meta-analysis of published studies concerning the diagnostic performance of ML-based MRI in predicting ALNM in breast cancer patients.
Introduction
Axillary lymph node metastasis (ALNM) determines the prognosis and treatment in breast cancer patients. Thus, accurate and reproducible detection of ALNM in these patients is crucial. Studies have been conducted on machine learning (ML) in predicting ALNM in breast cancer patients. However, the results are inconsistent, and no systematic literature search has been reported. Thus, the aim of the present study was to conduct a systematic review and meta-analysis concerning the diagnostic performance of ML-based MRI in predicting ALNM in breast cancer patients.Methods
A systematic search of PubMed, Embase, Web of Science, and the Cochrane library until 1 November 2020 was conducted to collect all relevant articles (Fig. 1). Two reviewers screened the papers and independently extracted characteristics and diagnostic outcomes for eligibility (Fig. 2). Sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio, and the area under the receiver operating characteristic curves (AUC) were pooled to quantify predictive accuracy (Fig. 3). Summary receiver operating characteristic curves were applied to evaluate the threshold effect. A fixed-effects model estimated the overall effect size, and the funnel plot was used to assess the publication bias. Data were processed with Review Manager 5.3, Stata14.0, and MetaDisc1.4.Results
Eleven studies assessing 1957 breast cancer patients (792 abnormal lymph nodes and 1338 normal lymph nodes) were included in the analysis [1-11] (Tables 1-2). The overall AUC for ML was 0.81 (95% confidence interval [CI]: 0.77-0.84). The pooled sensitivity and specificity were 0.75 (95% Cl 0.69–0.81) and 0.77 (95% Cl 0.73–0.81), respectively. Sensitivity analysis revealed that the 11 original articles had high stability and reliability. In subgroup analyses in the validation set, contrast-enhanced T1 (T1CE) with ML yielded higher sensitivity (0.80 vs. 0.66 vs. 0.68) and specificity (0.77 vs. 0.76 vs. 0.74) than did fat-suppressed T2 (T2-FS) and DWI. Also, ML performed better for 3T than for 1.5T, for which the pooled sensitivities were 0.76 vs. 0.75 and specificities were 0.79 vs. 0.76. Support vector machines (SVM) had higher specificity than did linear regression (LR) (0.83 vs. 0.73).Discussion
T2-weighted images (T2WI) can detect edema, hemorrhage, mucus, and cystic fluid, which can be valuable for evaluating breast masses. DWI is a commonly performed sequence, with promising results for evaluating breast lesions, as images can be obtained over a short time without contrast agents. However, most lesions on T2WI and DWI have inferior image quality and increased blurring and distortion, which can cause difficulty and inaccuracy in segmenting the lesions. T2-FS can identify lesion boundaries, whereas DCE-MRI with numerous scanning phases is sensitive to the change of perfusion and permeability of tissue vessels. Dong et al. reported that the predictive performance of ALNM combining T2-FS and DWI was better than that of T2-FS and DWI alone (AUC 0.863 vs 0.847 vs 0.847 in the training set, AUC 0.805 vs 0.770 vs 0.787 in the validation set). However, in this meta- analysis, the number of combined sequences was too small to permit reliable conclusions. Ren et al., Han et al., and Liu J et al. used the axial first phase of T1CE to predict ALNM respectively, which achieved AUC of 0.91, 0.78 and 0.81 in the validation set, respectively. Liu C et al., Liu M et al., and Cui et al. used the peak enhanced phase, which achieved an AUC of 0.85, 0.74 and 0.77 in the validation set. T1CE with ML yielded higher sensitivity and specificity than did DWI.Conclusions
ML demonstrated an excellent diagnostic performance for prediction of ALNM in breast cancer patients. MRI sequences, magnetic field strength, and algorithms were the main factors affecting the diagnostic performance of ML.Acknowledgements
No acknowledgement found.References
[1] Luo J, Ning Z, Zhang S, et al. Bag of deep features for preoperative prediction of sentinel lymph node metastasis in breast cancer[J]. Phys Med Biol, 2018,63(24):245014. [2] Dong Y, Feng Q, Yang W, et al. Preoperative prediction of sentinel lymph node metastasis in breast cancer based on radiomics of T2-weighted fat-suppression and diffusion-weighted MRI[J]. Eur Radiol, 2018,28(2):582-591. [3] Zhang X, Zhong L, Zhang B, et al. The effects of volume of interest delineation on MRI-based radiomics analysis: Evaluation with two disease groups[J]. Cancer Imaging, 2019,19(1). [4] Liu J, Sun D, Chen L, et al. Radiomics analysis of dynamic contrast-enhanced magnetic resonance imaging for the prediction of sentinel lymph node metastasis in breast cancer[J]. Frontiers in Oncology, 2019,9(SEP). [5] Spuhler K D, Ding J, Liu C, et al. Task-based assessment of a convolutional neural network for segmenting breast lesions for radiomic analysis[J]. Magnetic Resonance in Medicine, 2019,82(2):786-795. [6] Han L, Zhu Y, Liu Z, et al. Radiomic nomogram for prediction of axillary lymph node metastasis in breast cancer[J]. European Radiology, 2019,29(7):3820-3829. [7] Liu C, Ding J, Spuhler K, et al. Preoperative prediction of sentinel lymph node metastasis in breast cancer by radiomic signatures from dynamic contrast-enhanced MRI[J]. J Magn Reson Imaging, 2019,49(1):131-140. [8] Cui X, Wang N, Zhao Y, et al. Preoperative Prediction of Axillary Lymph Node Metastasis in Breast Cancer using Radiomics Features of DCE-MRI[J]. Sci Rep, 2019,9(1):2240. [9] Tan H, Gan F, Wu Y, et al. Preoperative Prediction of Axillary Lymph Node Metastasis in Breast Carcinoma Using Radiomics Features Based on the Fat-Suppressed T2 Sequence[J]. Academic Radiology, 2020,27(9):1217-1225. [10] Ren T, Cattell R, Duanmu H, et al. Convolutional Neural Network Detection of Axillary Lymph Node Metastasis Using Standard Clinical Breast MRI[J]. Clinical Breast Cancer, 2020,20(3):e301-e308. [11] Liu M, Mao N, Ma H, et al. Pharmacokinetic parameters and radiomics model based on dynamic contrast enhanced MRI for the preoperative prediction of sentinel lymph node metastasis in breast cancer[J]. Cancer Imaging, 2020,20(1):65.Figures
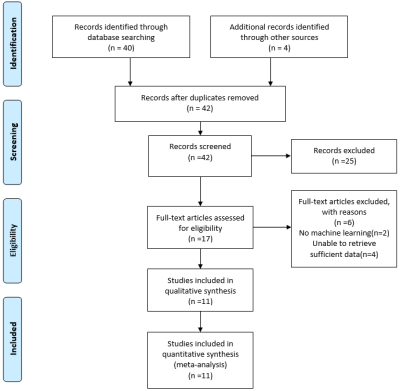
Fig. 1. Flow diagram of literature review
and study selection
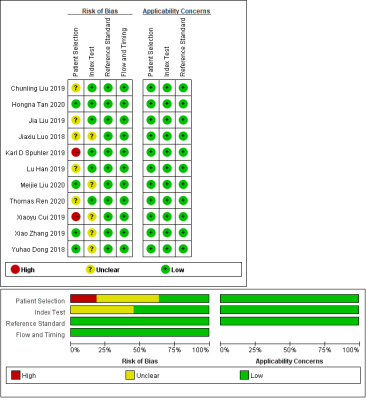
Fig. 2 Summary of risk of bias and
applicability concerns. Green represents low, yellow circle unclear, and red
high risk of bias.
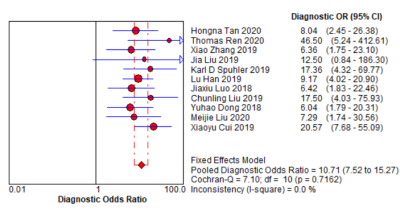
Fig. 3. Forest plot of single studies for
the pooled diagnostic odds ratio and 95% CI
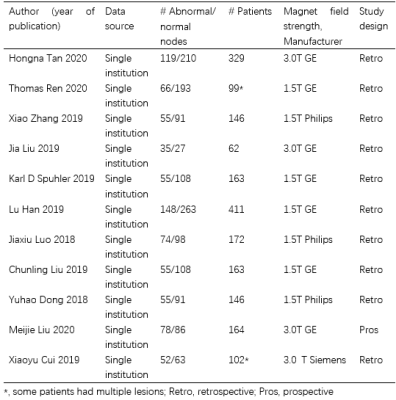
Table 1. Study and patient characteristics
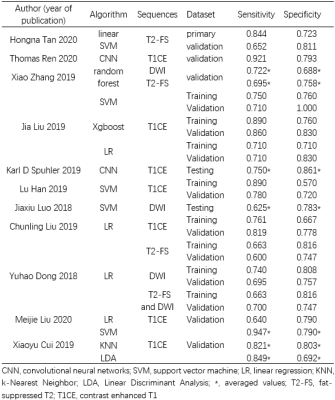
Table 2. Baseline characteristics of included
studies