1104
Calibration of myocardial perfusion quantification using a dedicated two-compartment cardiac phantom1School of Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom
Synopsis
Myocardial perfusion quantification by cardiovascular MR imaging has shown great promise in the detection of coronary artery disease. However, the lack of true standardization of methods across centers hinders the effective comparison and pooling of measurements. We sought to develop a novel cardiac phantom mimicking dynamic contrast exchange between two myocardial compartments and use it for the calibration of perfusion measured using three common quantification methods. Without calibration, perfusion measurements differed significantly between methods. Calibration led to accurate and non-significantly different measurements, suggesting that it could be an effective and reliable approach for the universal standardization of quantification methods.
Introduction
Measurement of myocardial blood flow (MBF) by first-pass cardiovascular MR perfusion has proven to be an effective and accurate marker of coronary artery disease.1-3 The usefulness of MBF quantification led to the development of a plethora of acquisition and post-processing pipelines across centers, only a handful of which have undergone independent validation. It is well acknowledged that the lack of standardization of perfusion methods hinders the effective comparison of absolute MBF measurements, and may render thresholds of disease and diagnostic accuracy values non-generalizable.4 Rather than standardizing quantification methods across centers, a more elegant and affordable solution would be the use of a dedicated physical standard to calibrate MBF measurements. The aim of this study was to present a physical standard for dynamic first-pass perfusion imaging incorporating a novel synthetic multi-compartmental myocardium. The reliability of the physical standard for perfusion calibration was evaluated on a 3T system.Methods
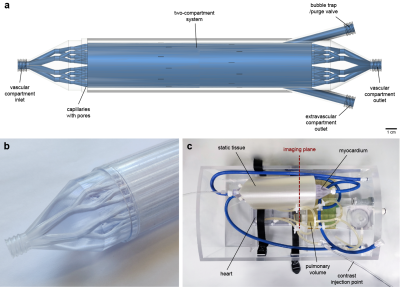
Myocardium and phantom designA spatially lumped two-compartment system simulating contrast exchange between a vascular and an extravascular compartment was designed and manufactured using high-precision industrial 3D printing (Figure 1). The vascular compartment is a set of parallel capillaries with 1440 sub-millimeter pores allowing diffusion of the contrast agent into the surrounding extravascular compartment. The vascular inlet and outlet resemble a vascular tree. This is an advanced version of a previously described prototype that represented a single functional unit,5 and is designed for compatibility with a hardware phantom system (Zürich MedTech AG, Zürich, Switzerland) (Figure 1c). The phantom is a torso-shaped box made with machined acrylic comprising of a four-chamber heart, a pulmonary volume, large thoracic vessels and coronary tubing for connecting the synthetic myocardium. Flow through the various components is achieved with a gear pump and flow controllers allowing independent control and monitoring of the cardiac output and the outflow from each myocardial compartment. In this experiment, only outflow from the vascular compartment was set so that the contrast exchange is passive and bidirectional, in accordance with the two-compartment exchange model (2CXM).6
Image acquisition
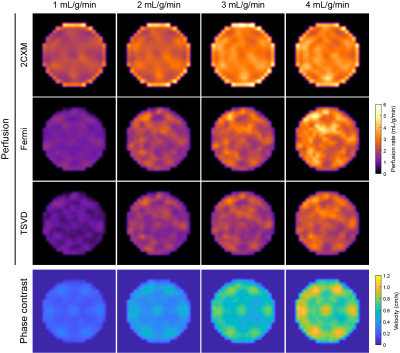
The phantom was scanned on a 3T Philips Achieva system equipped with a 32-channel cardiac phased-array coil (Philips Healthcare, Best, The Netherlands). An ECG-triggered saturation recovery spoiled gradient echo dual-sequence technique was used (TR 2.2 ms, TE 1.0 ms, saturation delay 100 ms, flip angle 15°, acquired resolution 2.6x2.6 mm2, slice thickness 8 mm),7 after injection of a contrast bolus at 0.05 mmol/kg (Gadovist, Bayer AG, Leverkusen, Germany). A low-resolution slice (parameters as above except saturation delay 23.5 ms, acquired resolution 2.6x5.3 mm2) was acquired at the level of the proximal aorta to allow sampling of the arterial input function.8 Three repeated scans for each of four reference myocardial MBF values (1-4 mL/g/min) were acquired. Furthermore, phase contrast imaging with matching spatial parameters was performed to assess the myocardium’s flow behavior.
Image and statistical analysis
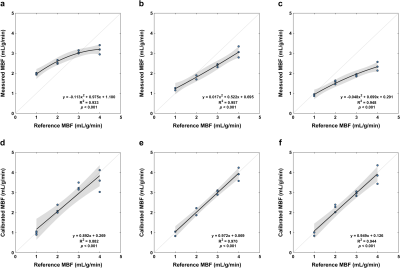
Pixel-wise MBF was estimated using tracer-kinetic modeling with the 2CXM, as well as the common Fermi function-constrained deconvolution and model-independent deconvolution using truncated singular value decomposition.6 2CXM analysis was stabilized by fixing the volumes of the two compartments based on their known values. Mean MBF was calibrated based on quadratic fitting of the measured and reference values, and MBF before and after calibration was compared between quantification methods using analysis of variance.
Results
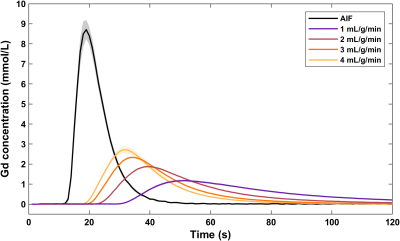
The synthetic myocardium produces tissue enhancement curves characteristic to a two-compartment perfusion model (Figure 2). At low MBF, the tracer arrival time in the myocardium is increased and contrast clearance is prolonged due to the slow exchange between the two compartments. In contrast, at high MBF the contrast rapidly perfuses and traverses the myocardium. For each quantification method, MBF increases with increasing reference values but with a nonlinear pattern (Figures 3, 4). MBF differed significantly between methods without calibration (F(1.260, 13.859) = 79.598, p < 0.001; all pairwise comparisons p < 0.001). Calibration led to a linear relationship between measured and reference MBF and no difference between methods (F(1.047, 11.522) = 0, p = 1).Discussion
While phantoms for the validation of CMR first-pass perfusion have been proposed in the past, this is the first solution that closely mimics the dynamics of gadolinium-based contrast agents within the myocardial tissue, performed so in a reliable and reproducible manner. The phantom was used for the assessment of the accuracy of three common methods for the measurement of MBF, uncovering a significant difference between measurements. The underestimation of MBF at higher reference values by all methods can be partially attributed to flow-related artifacts and signal saturation effects that may not be completely recovered by the conversion of signal intensity to gadolinium concentration. Irrespective of the measurement errors, phantom-based calibration alleviates the difference in MBF between methods and restores its linearity. Due to the compatibility of our phantom with other modalities, future work will focus on a multivendor and multimodality calibration of first-pass perfusion and its clinical translation.Conclusion
We developed a novel synthetic multi-compartmental phantom allowing reliable calibration of MBF measured with different quantification methods. The proposed solution could assist in the standardization of quantitative perfusion and the generalizability of ischemia thresholds, allowing effective comparison of measurements and eventually the clinical translation of the technique.Acknowledgements
XM and AC were funded by the European Metrology Programme for Innovation and Research (EMPIR) project 15HLT05 PerfusImaging, which is co-funded by the European Union's Horizon 2020 research and innovation programme and the EMPIR Participating States. XM was funded by the British Heart Foundation [TG/18/2/33768]. Further support was received by the Wellcome/EPSRC Centre for Medical Engineering [WT 203148/Z/16/Z] and the EPSRC Centre for Doctoral Training in Medical Imaging [EP/L015226/1], as well as the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust, the NIHR Cardiovascular MedTech Co-operative at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London and supported by the NIHR Clinical Research Facility (CRF) at Guy’s and St Thomas’. The views expressed are those of the authors and not necessarily those of the DoH, the NIHR, the NHS, the Wellcome Trust or the EPSRC.References
1. Villa ADM, Corsinovi L, Ntalas I, et al. Importance of operator training and rest perfusion on the diagnostic accuracy of stress perfusion cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2018;20(1):74.
2. Mordini FE, Haddad T, Hsu LY, et al. Diagnostic accuracy of stress perfusion CMR in comparison with quantitative coronary angiography: fully quantitative, semiquantitative, and qualitative assessment. JACC Cardiovasc Imaging. 2014;7(1):14-22.
3. Patel AR, Antkowiak PF, Nandalur KR, et al. Assessment of advanced coronary artery disease: advantages of quantitative cardiac magnetic resonance perfusion analysis. J Am Coll Cardiol. 2010;56(7):561-569.
4. Robinson AA, Salerno M, Kramer CM. Contemporary Issues in Quantitative Myocardial Perfusion CMR Imaging. Current Cardiovascular Imaging Reports. 2019;12(3):9.
5. Milidonis X, Ludovica B, Schaeffter T, et al. Validation of CMR perfusion using a synthetic multi-compartmental physical standard. Bellevue, WA: SCMR 22nd Annual Scientific Sessions; 2019.
6. Jerosch-Herold M. Quantification of myocardial perfusion by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2010;12:57.
7. Sanchez-Gonzalez J, Fernandez-Jimenez R, Nothnagel ND, et al. Optimization of dual-saturation single bolus acquisition for quantitative cardiac perfusion and myocardial blood flow maps. J Cardiovasc Magn Reson. 2015;17:21.
8. Franks R, Milidonis X, Schneider T, et al. Impact of the Arterial Input Sampling Location on CMR First-Pass Myocardial Perfusion Quantification. JACC Cardiovasc Imaging. 2020 (e-pub).
Figures