1103
High-resolution Spiral First-pass Myocardial Perfusion Imaging using DEep learning-based rapid Spiral Image REconstruction (DESIRE)1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Electrical and Computer Engineering, University of Virginia, Charlottesville, VA, United States, 3Medicine, University of Virginia, Charlottesville, VA, United States, 4Radiology, University of Virginia, Charlottesville, VA, United States
Synopsis
First-pass contrast-enhanced myocardial perfusion imaging is valuable for evaluating coronary artery disease (CAD). Spiral perfusion imaging techniques, using a motion-compensated L1-SPIRiT based reconstruction, are capable of whole-heart high-resolution perfusion imaging. However, this reconstruction is performed off-line and takes ~1 hour per slice. To address this limitation, we developed a DEep learning-based Spiral Image REconstruction technique (DESIRE) for spiral first-pass myocardial perfusion imaging, for both single-slice (SS) and simultaneous multi-slice (SMS) MB=2 acquisitions, to provide fast and high-quality image reconstruction and make rapid online reconstruction feasible. High image quality was demonstrated using the proposed technique for healthy volunteers and patients.
Introduction
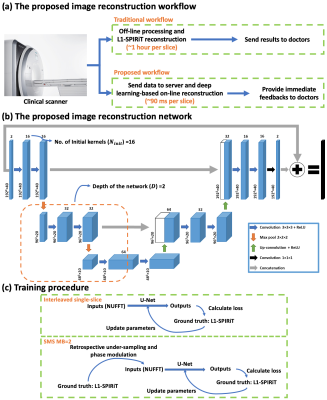
First-pass contrast-enhanced cardiac magnetic resonance (CMR) perfusion imaging, which is non-invasive and non-radioactive, has proven to be a valuable tool for evaluating patients with CAD1–4. Recently, we have developed spiral single-slice (SS) and simultaneous multi-slice (SMS) perfusion pulse sequence and the motion-compensated (SMS-Slice)-L1-SPIRiT reconstruction technique capable of whole-heart high-resolution perfusion imaging5–8. However, this compressed-sensing based image reconstruction technique is time-consuming and takes ~1 hour per slice, hence it can’t provide immediate feedback to doctors and impedes clinical translation. The goal of this study is to develop and evaluate a DEep learning-based Spiral Image REconstruction technique (DESIRE) for spiral first-pass myocardial perfusion imaging, for both SS and SMS MB=2 acquisitions, to provide fast and high-quality image reconstruction (Figure 1 (a)).Methods
Data Acquisition and Preprocessing SS and SMS golden-angle spiral perfusion data sets with 1.25×1.25 mm2 in-plane spatial resolution and whole-heart coverage (6-8 slices) were previously acquired from 18 healthy volunteers and 4 patients undergoing clinical studies on 3 T SIEMENS Skyra/Prisma scanners8,9. The in-plane acceleration factor for SS and SMS MB=2 was approximately 10 and 5, respectively.Before feeding the data to the network, coil-selection10, motion-correction11, and adaptive phase combination12 were performed on the NUFFT-gridded13 multi-coil image series at each slice location. Images were cropped into (Frames) to save memory. Each dynamic image series were normalized to 0-1.
Specifically, for SMS MB=2 acquisitions, to prevent slice-leakage artifact from being learned by the SMS network, the ground-truth data were SS L1-SPIRiT images from two slice locations, and the SMS network input images were the retrospective data from the two separate images with Hadamard SMS MB=2 phase modulation (Figure 1 (c))14.
156 slices from 20 subjects were used for training, and another 14 slices from 2 subjects were used for validation. Another 56 slices from 8 subjects with SS and 76 slices from 10 subjects with SMS MB=2 acquisitions were used for testing the performance.
Image Reconstruction Network Figure 1 (b) illustrates the proposed 3D U-Net15 based image reconstruction network. The inputs to the network were complex-valued under-sampled single-channel perfusion image series from a given slice location after coil-selection10, motion-correction11, and adaptive phase combination12. The real and imaginary values were concatenated into two channels. The outputs were the concatenated real and imaginary perfusion image series. SS and SMS network were trained separately where L1-SPIRiT reconstruction results served as the ground truth.
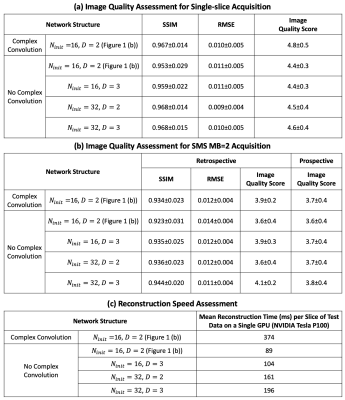
To compare networks, we set the network with and without complex convolution as the baseline. The performance of the network with respect to the number of kernels (Ninit) at initial layer and the depth of the network (D) was explored (Table 1). Furthermore, we sought to evaluate complex convolutional networks16 to preserve the phase information in the raw data to see if this could further improve reconstruction quality.
Experimental Setup The training of the baseline network was conducted using PyTorch on a single NVIDIA Tesla P100 GPU (12 GB memory). The training of the other networks was conducted on four P100 GPUs due to the memory limitation of a single GPU. All of the trainings were conducted for 150 epochs with an L1 loss (absolute error) and a batch size of 4. The shortest training time was baseline network which took ~8 hours, while the longest training time was complex convolution network which took ~20 hours. All of the testing experiments were conducted on a single P100 GPU.
Image Analysis Both structural similarity index (SSIM)17 and root mean square error (RMSE) for the SS and respective SMS MB=2 reconstructed using DEIRE were assessed with respect to the ground truth. Prospective SS and SMS images were graded by an experienced cardiologist (5, excellent; 1, poor).
Results
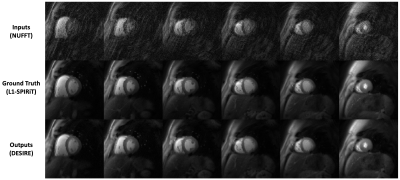
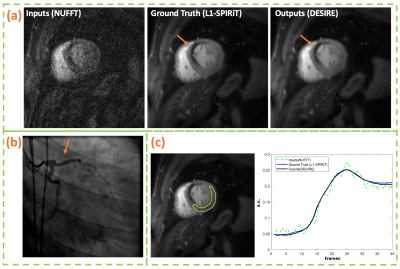
Figure 2 and 3 show examples from the test data for the SS (Figure 2) and SMS MB=2 (Figure 3) reconstructions using the proposed DESIRE technique. Good image quality was demonstrated.Figure 4 shows an example case using SS acquisition undergoing clinical stress spiral perfusion imaging. Good image quality and temporal fidelity with respect to the ground truth were demonstrated.
Table 1 (a) and (b) show image quality scores for both SS and SMS MB=2 with different network structures. Table 1 (c) shows the corresponding reconstruction time per slice of the test data on a NVIDIA Tesla P100 GPU, while the reconstruction time of using L1-SPIRiT with 80 iterations on an Intel Xeon CPU (2.40 GHz) was ~1 hour per slice. Increasing the depth and the number of initial kernels help improve the reconstruction performance. However, for SS with Ninit=32 , the performance of D=2 is similar to D=3, which indicates the max capacity of the reconstruction network is reached. Particularly, for the baseline network with complex convolution operations, the performance is improved. Scores from cardiologist showed a preference to networks with more initial kernels and those using complex convolutions, which was consistent with the SSIM and RMSE quantification.
Discussion and Conclusion
The proposed image reconstruction network (DESIRE) enabled rapid and high-quality image reconstruction for both SS and SMS MB=2 whole-heart ultra-high resolution first-pass spiral perfusion imaging. Further optimization of the SMS reconstruction network, such as incorporating the through-plane kernels is still required for optimal performance.Acknowledgements
This work was supported by NIH R01 HL131919 and Wallace H. Coulter
Foundation Grant.
References
1. Greenwood JP, Maredia N, Younger JF, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. The Lancet 2012;379:453–460.
2. Salerno M, Beller GA. Noninvasive Assessment of Myocardial Perfusion. Circ Cardiovasc Imaging 2009;2:412–424.
3. Jaarsma C, Leiner T, Bekkers SC, et al. Diagnostic Performance of Noninvasive Myocardial Perfusion Imaging Using Single-Photon Emission Computed Tomography, Cardiac Magnetic Resonance, and Positron Emission Tomography Imaging for the Detection of Obstructive Coronary Artery Disease: A Meta-Analysis. Journal of the American College of Cardiology 2012;59:1719–1728.
4. Lipinski MJ, McVey CM, Berger JS, Kramer CM, Salerno M. Prognostic Value of Stress Cardiac Magnetic Resonance Imaging in Patients With Known or Suspected Coronary Artery Disease: A Systematic Review and Meta-Analysis. Journal of the American College of Cardiology 2013;62:826–838.
5. Yang Y, Kramer CM, Shaw PW, Meyer CH, Salerno M. First-pass myocardial perfusion imaging with whole-heart coverage using L1-SPIRiT accelerated variable density spiral trajectories: Whole Heart Spiral Myocardial Perfusion Imaging. Magnetic Resonance in Medicine 2016;76:1375–1387.
6. Yang Y, Meyer CH, Epstein FH, Kramer CM, Salerno M. Whole‐heart spiral simultaneous multi‐slice first‐pass myocardial perfusion imaging. Magnetic Resonance in Medicine 2019;81:852–862.
7. Wang J, Yang Y, Zhou R, Jacob M, Weller DS, Salerno M. SMS Slice L1-SPIRiT: auto-calibrated image reconstruction for spiral simultaneous multi-slice first-pass perfusion imaging with 1.25 mm resolution and whole heart coverage at 3T. In: Proceedings of the SCMR 23rd Annual Scientific Sessions, Orlando, Florida, USA, 2020.
8. Wang J, Yang Y, Zhou R, et al. High resolution spiral simultaneous multi‐slice first‐pass perfusion imaging with whole-heart coverage at 1.5 T and 3 T. In: Proceedings of the 28th Annual Meeting of ISMRM, 2020. p 1313.
9. Yang Y, Kramer C, Salerno M. Whole heart First-pass spiral perfusion imaging with 1.25mm resolution at 3T. In: Proceedings of the 26th Annual Meeting of ISMRM, Paris, France, 2018. p 3321.
10. Zhou R, Yang Y, Mathew RC, et al. Free-breathing cine imaging with motion-corrected reconstruction at 3T using SPiral Acquisition with Respiratory correction and Cardiac Self-gating (SPARCS). Magnetic Resonance in Medicine 2019;82:706–720.
11. Zhou R, Huang W, Yang Y, et al. Simple motion correction strategy reduces respiratory-induced motion artifacts for k-t accelerated and compressed-sensing cardiovascular magnetic resonance perfusion imaging. J Cardiovasc Magn Reson 2018;20:6.
12. Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. Magnetic Resonance in Medicine 2000;43:682–690.
13. Fessler JA, Sutton BP. Nonuniform fast Fourier transforms using min-max interpolation. IEEE Transactions on Signal Processing 2003;51:560–574.
14. Souza SP, Szumowski J, Dumoulin CL, Plewes DP, Glover G. SIMA: simultaneous multislice acquisition of MR images by Hadamard-encoded excitation. J Comput Assist Tomogr 1988;12:1026–1030.
15. Ronneberger O, Fischer P, Brox T. U-Net: Convolutional Networks for Biomedical Image Segmentation. arXiv:1505.04597 [cs] 2015.
16. Cole E, Cheng J, Pauly J, Vasanawala S. Analysis of Deep Complex-Valued Convolutional Neural Networks for MRI Reconstruction. :10.
17. Zhou Wang, Bovik AC, Sheikh HR, Simoncelli EP. Image quality assessment: from error visibility to structural similarity. IEEE Transactions on Image Processing 2004;13:600–612.
Figures