1083
Evaluating the Effects of Motion Compensation to IVIM Fitting in In-Vivo DW-MRI of the Muscle1Cardiovascular Research Center, Cardiology Division, Massachusetts General Hospital, Charlestown, MA, United States, 2Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 3Harvard Medical School, Boston, MA, United States
Synopsis
In order to acquire diffusion-weighted images (DWI) of the heart, motion compensated (M2) techniques are needed. However, these reduce the sensitivity to perfusion (D*) and hence hamper the accurate estimation of IVIM parameters. Here, we evaluate the effects of M2 to the IVIM estimates in the lower leg muscle and assess feasibility of an approach to combine DWI acquired with and without M2 to fit IVIM models. Our results show that M2 underestimates D* in the IVIM model. On the other hand, the combined approach resulted in IVIM parameter estimates similar to those obtained with standard DW MR.
Introduction
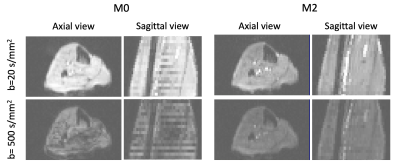
Intravoxel incoherent motion (IVIM) makes it possible to estimate the capillary blood flow (CBF) in the tissue from a series of diffusion weighted (DW) MR images acquired for multiple b-values [1,2]. CBF is important for cardiologists since it provides information on various physiological and pathophysiological states of the cardio-vasculature in the skeletal muscle as well as the myocardial tissue. Moreover, the recent introduction of motion compensated techniques to DW-MRI has made possible to successfully overcome the inherent bulk motion sensitivity of DW-MRI [3]. These techniques are particularly important when acquiring DWI with high b-values since bulk motion would otherwise introduce large artifacts. However, they also have been shown to have reduced sensitivity to the perfusion signal in ex-vivo experiments [4]. See Figure 1 for an illustration of these effects. Consequently, it is not clear what is the best approach to acquire DWI of dynamic muscle tissue for IVIM fitting. One potential solution to balance motion and low sensitivity to perfusion is to acquire the DWI with low b-value without using motion compensation and enabling it for larger b-values. Hence, the objective of this work is two-fold. It aims to first, compare the results of IVIM fitting to in-vivo DWI of calf acquired with and without motion compensation, and second, assess the feasibility of combining the DWI of these two acquisitions.Methods
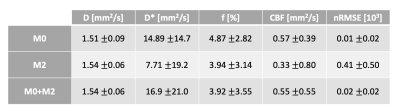
IVIM-MRI of the calf was performed on 5 healthy volunteers (mean age 39 years old, 1 female) in a 3T Prisma MRI scanner with a dedicated single-channel leg coil. The acquisition was performed with two DW-MRI sequences using 2DRF zoomed EPI readout, one with 2nd order motion compensation (M2) [3] and another without it (M0). DWI were acquired for 10 b-values (0, 10, 20, 30, 50, 80, 120, 200, 300, 500 s/mm2) over 3 orthogonal directions x10 averages. Image resolution was 2.5x2.5x5mm3, matrix size 64x52, 24 slices, pixel bandwidth 2005 Hz/pixel, TE=48/72ms for M0 and M2, respectively. After acquisition, the calf was segmented into 5 muscle groups (Gastrocnemius, Soleus, Tibialis posterior, Tibialis anterior and ‘other muscles’) and the DW signal was averaged over gradient directions using the geometric mean average and over voxels within each muscle group using the median. M0 and M2 average signals were combined to form a joined (M0+M2) signal with data from M0 for b<200s/mm2 and M2 for b>200s/mm2. Before combination, the M0 data was normalized to compensate for amplitude differences between M0 and M2. The normalization constant was further used to estimate T2* values. The IVIM model was then fitted to the average M0, M2 and M0+M2 signals using a two-stage approach [5]. This method first separately fitted the diffusion (D) and perfusion (D*) parameters to the DWI with b>200s/mm2 and b<200s/mm2, respectively. Afterwards, it used a non-linear approach initialized with the results from the previous step to jointly estimate D, D* and perfusion fraction (f). The b=0s/mm2 images for M2 were discarded during fitting to avoid bias in the estimation of perfusion. Results for M0, M2 and M0+M2 were compared through the difference between the IVIM parameter estimates (D, D* and f), the resulting CBF (measured as fxD*) and the goodness of fit of the IVIM model reported as the normalized-root-mean-squared error (nRMSE). Statistical comparisons were done with Wilcoxon rank test and p-value 0.05.Results
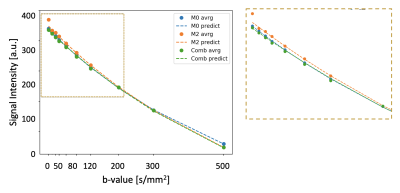
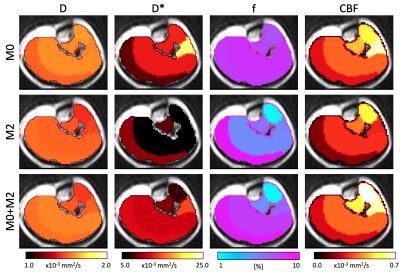
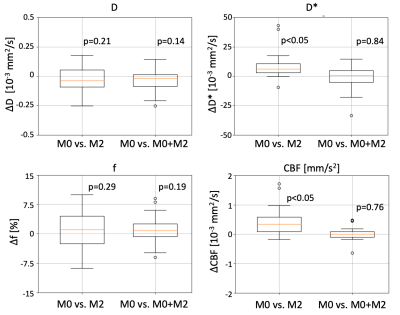
The average normalization constant between M0 and M2 was 0.55+/-0.05, which corresponded to a typical T2* value for the muscle (35.6+/-8.5 sec) [6]. Figure 2 shows representative average intensity curves over b-value for M0, M2 and M0+M2 signals. M2 presents a discontinuity at b=0 s/mm2. On the other hand, the combined M0+M2 signal does not present apparent discontinuities at the point of combination (b=200 s/mm2) nor at b=0. M0, M2 and M0+M2 have similar intensities although these differ for small b-values, where perfusion effects are dominant. The IVIM parameters estimated in each muscle group of a representative subject are shown in Figure 3 and the IVIM parameter averages are summarized in Table 1. The results estimated with M0 are in concordance with previous reports [7,8]. On the other hand, M2 underestimated CBF. Figure 4 shows a boxplot of the differences between M0 vs. M2 as well as M0 vs. M0+M2. The parameters D*, f and CBF presented large differences between M0 and M2, although only the difference of D* and CBF were significant. On the other hand, all parameters estimated using the M0+M2 signal showed no statistical differences with M0.Conclusions
Motion-compensated DW-MRI sequences introduce errors in the estimation of the perfusion parameters in IVIM, which can be corrected with the adequate combination of DWI acquired with and without motion compensation. The differences in signal between the two acquisitions can be corrected with post-processing techniques or prospectively by adjusting TE in the M0 acquisition. This approach could potentially provide accurate IVIM fits in the presence of bulk motion and, hence, contrast-free estimates of muscle perfusion and capillary blood flow during a dynamic state.Acknowledgements
This work was partially funded by the National Institutes of Health awards R01HL135242 and R01HL151704.References
[1] D. Le Bihan and R. Turner, “The capillary network: a link between ivim and classical perfusion,” Magn. Reson. Med., vol. 27, no. 1, pp. 171–178, Sep. 1992.
[2] D. Lebihan, E. Breton, D. Lallemand, M. L. Aubin, J. Vignaud, and M. Laval-Jeantet, “Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging.,” Radiology, vol. 168, no. 2, pp. 497–505, Aug. 1988.
[3] C. T. Nguyen et al., “Free‐breathing diffusion tensor MRI of the whole left ventricle using second‐order motion compensation and multitasking respiratory motion correction,” Magn. Reson. Med., in press. Nov. 2020.
[4] G. R. Spinner, C. T. Stoeck, L. Mathez, C. von Deuster, C. Federau, and S. Kozerke, “On probing intravoxel incoherent motion in the heart‐spin‐echo versus stimulated‐echo DWI,” Magn. Reson. Med., vol. 82, no. 3,, Apr. 2019.
[5] H. Farooq et al., “Microstructure Imaging of Crossing (MIX) White Matter Fibers from diffusion MRI,” Sci. Rep., vol. 6, no. September, pp. 1–9, 2016.[6] C. Roy et al., “Age and sex corrected normal reference values of T1, T2 T2∗and ECV in healthy subjects at 3T CMR,” J. Cardiovasc. Magn. Reson., vol. 19, no. 1, p. 72, Sep. 2017.
[7] A. Ogura, H. Sotome, A. Asai, and A. Fuju, “Evaluation of capillary blood volume in the lower limb muscles after exercise by intravoxel incoherent motion,” Radiol. Medica, vol. 125, no. 5, pp. 474–480, May 2020.
[8] F. Adelnia et al., “Diffusion‐weighted MRI with intravoxel incoherent motion modeling for assessment of muscle perfusion in the thigh during post‐exercise hyperemia in younger and older adults,” NMR Biomed., vol. 32, no. 5, p. e4072, May 2019.
Figures