1080
Simultaneous Effects of tDCS on Cerebral Blood Flow and Metabolic Response in Healthy Controls1Department of Radiology, NYU Grossman School of Medicine, New York City, NY, United States, 2Soterix Medical Inc., New York City, NY, United States, 3Department of Biomedical Engineering, City College of New York, New York City, NY, United States
Synopsis
The underlying biological mechanisms of transcranial direct current stimulation (tDCS) remain relatively uncharacterized. In this report, MRI measures for cerebral blood flow (CBF) and cerebral metabolic rate of oxygen (CMRO2) of healthy controls was acquired before and during tDCS to better understand real-time neuronal response. We observed a significant increase in global CBF suggesting an increase of neuronal activity during compared to before tDCS. CMRO2 showed an increase of ~7% during tDCS compared to before tDCS. We suggest that the reported cortical excitability increase may also serve as a potential biomarker for neuronal reactivity or plasticity in many neurodegenerative diseases.
Introduction
Transcranial direct current stimulation (tDCS) is a safe, well-tolerated, and noninvasive brain stimulation that modulates cortical excitability by applying weak electrical currents to implement direct transcranial polarization[1].The use of tDCS in neuronal stimulation has gained a significant increase in popularity with positive effects for a number of neurological diseases. Despite its positive clinical findings, the underlying in vivo physiological underpinnings of tDCS remain largely unclear. There is a gap of knowledge regarding how externally applied tDCS modifies brain blood flow and oxygen metabolic properties even in healthy subjects. The purpose of this study is therefore to investigate the simultaneous effects that tDCS has on cerebral blood flow (CBF) and venous oxygenation (Yv) in a cohort of healthy subjects. Consequently, a measure of the metabolic rate of oxygen consumption (CMRO2) can be extrapolated, giving a better understanding of the neuronal reactivity to the stimulation. The higher CMRO2 change reflects higher neuronal response with decreased Yv or increased oxygen consumption during stimulation versus pre-stimulation. The ability to conclusively understand the effects that tDCS has on neuronal response related to blood flow and oxygen metabolism could offer an innovative translation of the treatment into a spectrum of neurological diseases.Methods
As part of a larger study, 23 healthy subjects (age: 36.2±15 years, 14 female) were recruited to complete a simultaneous tDCS and MRI scan. All participants were recruited after study-specific screening, excluding for any neurological diseases. All studies were performed by following protocols approved by the institutional review board. MRI was performed using a 3T with a 64 channels head coil. For testing the real time tDCS effects, a MR-compatible tDCS device (Soterix Medical) was used to acquire simultaneous MRI data before and during the stimulation. During the MRI scanning session subjects received left anodal dorsolateral prefrontal cortex stimulation for 15 minutes with current at 2.0 mA. Brain structural data was acquired with 3D T1 MPRAGE images, T2-Relaxation-Under-Spin-Tagging (TRUST) was acquired to assess venous blood oxygenation (Yv) at the lower segment of superior sagittal sinus above the confluence [2]. Finally Phase contrast (PC) MRI was acquired from 4 major brain feeding arteries (bilateral internal carotid arteries and vertebral arteries) to estimate the total cerebral blood flow (CBF) normalized for individual intracranial volume. From both of these measurements, CMRO2 was extrapolated using equations reported previously [3]. The statistical analysis of the results involved a paired t-test to assess the significance of difference in CBF, Yv and CMRO2 between pre and during stimulation.Results and Discussion
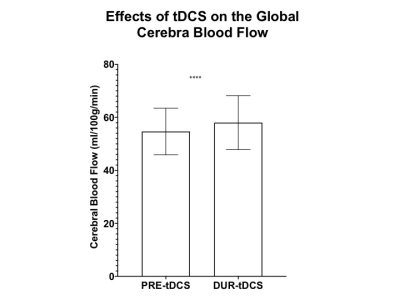
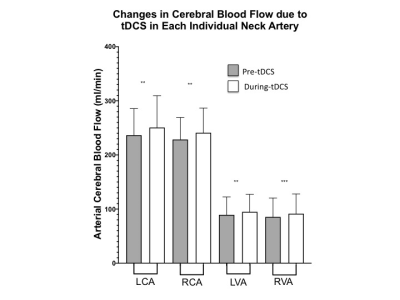
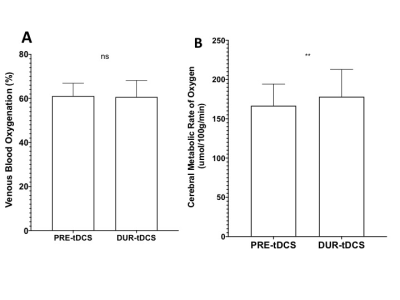
Firstly, the neuronal response to tDCS was investigated by comparing global CBF levels before and during the stimulation. The results show significantly higher total CBF during tDCS (58.0±10.2 ml/100g/min) as compared to pre-tDCS (54.7±8.7 ml/100g/min; p<0.001). This is in line with other studies reporting global and regional CBF increase[4-6]and also supports our hypothesis that tDCS stimulates neurons into more active states and therefore causes an increase in blood supply through neurocoupling mechanisms. To address the specificity of the effects that tDCS could have on blood flow, the CBF from individual neck feeding arteries was analysed. As hypothesized, both left and right internal carotid arteries showed a similar significant increase in CBF during tDCS (left:250.7±58.8 ml/min; right: 241.1±45.8 ml/min) and pre-tDCS (left:236.4±49.3; right:228.5±40.8 ml/min; p<0.005). We propose that tDCS’s effects on neuronal activity are not limited to only the region of the electrode application (frontal lobes in this study), but they ripple throughout the brain, causing the reported global increase of CBF. In fact, an average ~7% increase (p<0.005) in CBF during the stimulation was also observed in the vertebral arteries, even if they mainly provide blood supply to the posterior structures of the brain.Subsequently, CMRO2 was observed to increase from 166.6±27.6 μmol/100g/min before stimulation to 178.1±34.8 μmol/100g/min during tDCS (p<0.005) although no significant differences of Yv were found between pre-tDCS (61.1±5.8%) and during-tDCS (60.7±7.5%). The results suggest the CMRO2 change is predominantly determined by CBF change not Yv, since Yv change can be antithetical by two complicated scenarios: decrease due to increased neuronal activity and increase due to over supply from increased neurovascular coupling activity (BOLD effect). However, these findings support our hypothesis that tDCS induced changes in cortical excitability promoting neuronal O2 consumption and this may serve as a potential biomarker of brain reserve for predicting behavioral responsiveness to tDCS.Conclusion
From the results of this preliminary study, it is evident that tDCS has direct effects on the neuronal excitability of healthy subjects that are not limited to the region stimulated but perpetuate throughout the brain. This consequently promotes an increase in cerebral blood supply and oxygen consumption in order to support such an increase of neuronal activity. It is also noteworthy to highlight how the neuronal response matches the effects of the external stimulus in order to maintain constant homeostatic equilibrium. These effects in healthy subjects offer an opportunity to understand the full extent of tDCS’ therapeutic potential with potential applications in several neurodegenerative diseases.Acknowledgements
This study was funded by National Institute of Health (NIH/NICHD: R21 HD094424)References
1. Nitsche, M.A., Cohen, L.G., Wassermann, E.M., Priori, A., Lang, N., Antal, A., Paulus, W., Hummel, F., Boggio, P.S., Fregni, F. , Pascual-Leone, A., Transcranial direct current stimulation: state of the art 2008.Brain stimulation, 2008.1(3): p. 206-223.
2. Lu H, Ge Y., Quantitative evaluation of oxygenation in venous vessels using T2-Relaxation-Under-Spin-Tagging MRI. .Magn Reson Med, 2008. 60(2): p. 357-363.
3. Ge, Y., Zhang, Z., Lu, H., Tang, L., Jaggi, H., Herbert, J., Babb, J.S., Rusinek, H., Grossman, R.I., Characterizing brain oxygen metabolism in patients with multiple sclerosis with T2-relaxation-under-spin-tagging MRI. .Journal of Cerebral Blood Flow & Metabolism, , 2012. 32(3 ): p. 403-412.
4. Lang, N., Siebner, H.R., Ward, N.S., Lee, L., Nitsche, M.A., Paulus, W., Rothwell, J.C., Lemon, R.N., Frackowiak, R.S., , How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain?European Journal of Neuroscience, 2005. 22(2): p. 495-504.
5. Stagg, C.J., Lin, R.L., Mezue, M., Segerdahl, A., Kong, Y., Xie, J., Tracey, I., Widespread modulation of cerebral perfusion induced during and after transcranial direct current stimulation applied to the left dorsolateral prefrontal cortex.Journal of Neuroscience, 2013. 33(28): p. 11425-11431.
6. Zheng, X., Alsop, D.C., Schlaug, G.,, Effects of transcranial direct current stimulation (tDCS) on human regional cerebral blood flow.Neuroimage, 2011.58(1): p. 26-33.
Figures