1074
Do You Double-Dose? Clinical Validation of Single-Dose, Dual-Echo Perfusion Protocols in Glioma Patients1Neuroimaging Research, Barrow Neurological Institute, Phoenix, AZ, United States, 2Mayo Clinic Arizona, Phoenix, AZ, United States, 3Neuroradiology, Barrow Neurological Institute, Phoenix, AZ, United States
Synopsis
Relative cerebral blood volume (rCBV) obtained from dynamic susceptibility contrast (DSC) MRI is adversely impacted by contrast agent leakage effects in enhancing brain tumors, often necessitating multiple contrast doses. The purpose of this study is to compare dual-echo rCBV maps (single-dose) with standard rCBV maps (double-dose) in patients with high-grade gliomas using commercially available software. High agreement was observed between rCBV maps with single- and double-doses, with substantial flexibility across pulse sequence parameters. This protocol could be used to lower costs and contrast dose in clinical settings, as well as eliminate variability in perfusion methods related to contrast timing schemes.
Introduction
DSC MR imaging is increasingly used in the clinical management of patients with high-grade gliomas (HGG) to assess tumor angiogenesis and underlying vasculature. Unfortunately, contrast agent leakage effects can limit the reliability of DSC-derived rCBV measurements. Although specific single-dose protocols have shown good performance [1], the best overall performance based on accuracy and precision remains a double-dose protocol [2], where a full preload dose is given 5–10 minutes before dynamic imaging to reduce T1 leakage effects [3,4]. However, this double bolus strategy has multiple drawbacks, including higher cost, larger contrast dose, and the potential for increased protocol variability, due to multiple timed injections. A single bolus scheme would simplify and facilitate standardized clinical applications, while reducing contrast agent dose. We recently showed that dual-echo DSC can provide high rCBV accuracy in the absence of a preload dose across a wide range of intracranial neoplasms [5], which matched prior simulations using a digital reference object [6]. The purpose of this study is to validate those findings in a clinical setting, using FDA-approved commercial software. We hypothesize that the diagnostic accuracy of single-bolus dual-echo perfusion will be similar to the validated double-dose standard and this protocol could be used to lower costs and contrast dose in HGG patients, as well as eliminate variability in perfusion methods related to contrast timing schemes [7].Methods
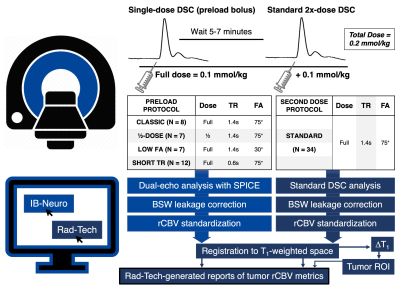
A total of 34 patients with HGG were included. Patients underwent a conventional MRI protocol with a double-bolus DSC perfusion scan at 3T (Philips Ingenia). Two contrast doses of gadobutrol were given during consecutive DSC-MRI acquisitions. During the first bolus, a dual-echo DSC protocol was performed (TE1/TE2 = 7.3/33.3ms, TR, FA, and dose combinations can be found in Figure 1). After a delay of 6 minutes, a full dose (0.1 mmol/kg) contrast bolus was given during a standard DSC acquisition (TE = 30ms, TR = 1.4s, FA = 75° for all 34 patients). This simultaneously permits evaluation of the single bolus dual-echo technique and serves as preload for the double bolus standard technique. Analysis was performed using FDA-approved commercial software from Imaging Biometrics (IB, Elm Grove, WI). The dual-echo perfusion maps were analyzed with IB-Neuro, using SPICE technology for analysis [8], contrast agent leakage correction using the standard Boxerman-Schmainda-Weisskoff (BSW) method [9], and standardization of perfusion maps [10]. Single-echo perfusion maps were obtained as part of the IB-RadTech workflow, which includes BSW correction, image standardization, registration to T1-weighted anatomical space, and computation of ΔT1 maps for extraction of tumor enhancing regions-of-interest (ROIs). A second custom IB-RadTech was then implemented to match the registration steps across both perfusion scans. Reports generated by IB-RadTech provided relative cerebral blood volume (rCBV) in the tumor ROIs for both dual-echo and single-echo acquisitions. Tumor rCBV (mean and standard deviation (SD), as a proxy for intratumor variability) were compared between acquisitions using a paired t-test with step-wise Holm-Bonferroni correction for multiple comparisons.Results
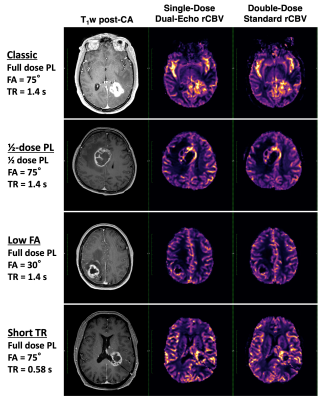
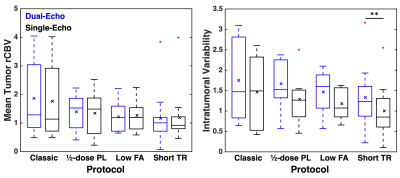
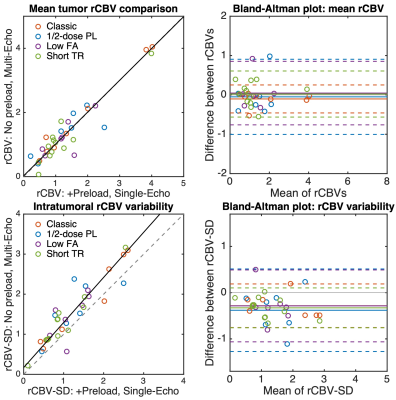
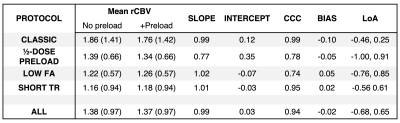
Dosing protocols and software workflow are shown in Figure 1. Figure 2 shows a representative T1-weighted post-contrast image, dual-echo rCBV, and single-echo rCBV from each protocol. The rCBV maps from each injection demonstrate high similarity across protocols, despite varying protocols. Mean tumor rCBV for both injections are shown in boxplots in Figure 3, as well as rCBV-SD, for each protocol. Tumor rCBV and rCBV-SD were similar across injection protocols, although intratumor variability was significantly different for the short TR protocol (p = 0.002). Correlation and Bland-Altman plots for tumor rCBV (mean and SD) across all 34 subjects are shown in Figure 4. The group-wise mean (SD) tumor rCBV, slope and intercept, and CCC values are shown in Table 1, along with bias and limits of agreement (LoA) for each protocol.Discussion and Conclusions
These results show that single-bolus dual-echo rCBV is comparable to standard (double-bolus) rCBV using commercial analysis software in a clinical setting, verifying our previous simulation results and in vivo studies. Other approaches to reducing contrast agent dosing include low-flip angle acquisitions [1], which have shown excellent consistency with the standard protocol, and split-dosing protocols [11], which may reduce multi-site concordance. However, dual-echo protocols have a flexibility advantage in acquisition parameters compared to other single-dose protocols, as pulse sequence parameters such as TR and FA have little impact on the resulting rCBV. Therefore, dual-echo protocols may decrease costs, reduce contrast dosage, and increase consistency of rCBV in HGG patients while allowing for protocol flexibility; additionally, it could lead to more widespread use of DSC, as it requires no extra contrast agent beyond standard dosing. These findings contribute to a trove of recent results suggesting that optimized brain tumor perfusion protocols can provide high reproducibility and less contrast dosage while maintaining rCBV accuracy [1,2,5,6,12]. This would be particularly valuable in clinical trials, where a simplified protocol would increase reproducibility across sites, and the single bolus would allow perfusion imaging to be performed within the parameters of the standardized Brain Tumor Imaging Protocol [13].Acknowledgements
This work was supported by the Foundation of the ASNR Comparative Effectiveness Grant, Arizona Biomedical Research Commission (ADHS16-162414), NIH/NCI CA213158, NS082609, CA221938, CA220378, and Philips Healthcare. The authors thank Timothy Dondlinger and Michael Schmainda for their help with the custom IB workflows.References
[1] Schmainda KM, Prah MA, Hu LS, Quarles CC, Semmineh N, Rand SD, et al. Moving Toward a Consensus DSC-MRI Protocol : Validation of a Low – Flip Angle Single-Dose Option as a Reference Standard for Brain Tumors. AJNR Am J Neuroradiol 2019;40:626–33. doi:10.3174/ajnr.A6015.
[2] Semmineh NB, Stokes AM, Bell LC, Boxerman JL, Quarles CC. A Population-Based Digital Reference Object (DRO) for Optimizing Dynamic Susceptibility Contrast (DSC)-MRI Methods for Clinical Trials. Tomography 2017;3:41–9. doi:10.18383/j.tom.2016.00286.
[3] Welker K, Boxerman J, Kalnin A, Kaufmann T, Shiroishi M, Wintermark M, et al. ASFNR recommendations for clinical performance of MR dynamic susceptibility contrast perfusion imaging of the brain. AJNR Am J Neuroradiol 2015;36:E41-51. doi:10.3174/ajnr.A4341.
[4] Boxerman JL, Quarles CC, Hu LS, Erickson BJ, Gerstner ER, Smits M, et al. Consensus Recommendations for a Dynamic Susceptibility Contrast MRI Protocol for Use in High-Grade Gliomas. Neuro Oncol 2020. doi:10.1093/neuonc/noaa141.
[5] Nespodzany A, Alhilali LM, Hu LS, Baxter LC, Quarles CC, Stokes AM. Evaluation of Single Bolus, Multi-Echo Dynamic Susceptibility Contrast Protocols in Brain Tumor Patients. Proc. 27th Annu. Meet. ISMRM, 2019, p. 0394.
[6] Stokes AM, Semmineh NB, Nespodzany A, Bell LC, Quarles CC. Systematic assessment of multi-echo dynamic susceptibility contrast MRI using a digital reference object. Magn Reson Med 2020;83. doi:10.1002/mrm.27914.
[7] Hu LS, Baxter LC, Pinnaduwage DS, Paine TL, Karis JP, Feuerstein BG, et al. Optimized preload leakage-correction methods to improve the diagnostic accuracy of dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging in posttreatment gliomas. Am J Neuroradiol 2010;31:40–8.
[8] Paulson ES, Prah DE, Schmainda KM. Spiral Perfusion Imaging With Consecutive Echoes (SPICE) for the Simultaneous Mapping of DSC- and DCE-MRI Parameters in Brain Tumor Patients: Theory and Initial Feasibility. Tomography 2016;2:295–307. doi:10.18383/j.tom.2016.00217.
[9] Boxerman JL, Schmainda KM, Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol 2006;27:859–67.
[10] Prah MA, Stufflebeam SM, Paulson ES, Kalpathy-Cramer J, Gerstner ER, Batchelor TT, et al. Repeatability of standardized and normalized relative CBV in patients with newly diagnosed glioblastoma. Am J Neuroradiol 2015;36:1654–61. doi:10.3174/ajnr.A4374.
[11] Bell LC, Semmineh N, An H, Eldeniz C, Wahl R, Schmainda KM, et al. Evaluating Multisite rCBV Consistency from DSC-MRI Imaging Protocols and Postprocessing Software Across the NCI Quantitative Imaging Network Sites Using a Digital Reference Object (DRO). Tomogr (Ann Arbor, Mich) 2019;5:110–7. doi:10.18383/j.tom.2018.00041.
[12] Semmineh NB, Bell LC, Stokes AM, Hu LS, Boxerman JL, Quarles CC. Optimization of Acquisition and Analysis Methods for Clinical Dynamic Susceptibility Contrast MRI Using a Population-Based Digital Reference Object. Am J Neuroradiol 2018;39. doi:10.3174/ajnr.A5827.
[13] Ellingson BM, Bendszus M, Boxerman J, Barboriak D, Erickson BJ, Smits M, et al. Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro Oncol 2015;17:1188–98. doi:10.1093/neuonc/nov095.
Figures