1073
Comparison of Gadolinium- and Iron-Oxide-based Perfusion Imaging Metrics in Glioblastoma1Neuroimaging Research, Barrow Neurological Institute, Phoenix, AZ, United States, 2Neuro-Oncology, Barrow Neurological Institute, Phoenix, AZ, United States, 3Neuroradiology, Barrow Neurological Institute, Phoenix, AZ, United States
Synopsis
Relative cerebral blood volume (rCBV) maps are adversely impacted by contrast agent leakage effects when acquired using gadolinium-based contrast agents, and these effects can be corrected using standard leakage correction methods. In a cohort of high-grade glioma patients, we compared leakage-corrected rCBV maps acquired using gadolinium-based contrast agents to rCBV acquired using an intravascular iron-oxide-based contrast agent. The rCBV maps from both contrast agents were similar, with high voxel-wise agreement, suggesting that leakage effects are appropriately removed from rCBV maps using standard leakage correction methods.
Introduction
Measures of relative cerebral blood volume (rCBV) from dynamic susceptibility contrast (DSC) MRI are widely used in neuro-oncology to differentiate glioma grades (1), distinguish tumor recurrence from post-treatment effects (2), and predict treatment response (3). Despite this potential impact on clinical care, DSC methods that use gadolium-based contrast agents (GBCA) are known to be hampered by contrast agent leakage effects, which lead to rCBV inaccuracies. These effects are mediated using leakage correction methods (4–7), some of which have been validated in preclinical studies against a reference standard contrast agent that does not leak out of the vasculature (4,8). However, no study to date has validated the accuracy of leakage-corrected rCBV in patient populations. The purpose of this study is to compare rCBV measures acquired during GBCA injections, with comparison to an intravascular iron-oxide-based (Fe) contrast injection as a reference standard.Methods
Patients with high-grade brain tumors undergoing a double-bolus DSC perfusion scan with GBCA at 3T (Philips) were recruited for this ongoing study (n = 4). As part of the standard of care, the first GBCA injection (gadobutrol, 0.1 mmol/kg) was performed during a dual-echo acquisition; after 6 minutes, a second GBCA injection (gadobutrol, 0.1 mmol/kg) was performed during a single-echo acquisition. The latter serves as the clinical standard of care with a preload dose. Following the T1-weighted post-contrast imaging, study participants underwent an injection of iron-oxide-based contrast agent (ferumoxytol, 2 mg/kg) during a dual-echo acquisition. The dual-echo acquisitions (1st and 3rd injection) were acquired with TE1/TE2 = 7.3/33.3ms, TR = 582 ms, FA = 75° for 2.14 minutes. The single-echo acquisition (2nd injection) followed our standard DSC protocol: TE = 30ms, TR = 1.4s, FA = 75° for 2.5 minutes. Leakage correction was performed using the standard Boxerman-Schmainda-Weisskoff (BSW) method for both GBCA injections (1). Maps of rCBV, obtained from trapezoidal integration over the first 120s, were compared across all three bolus injections, with the iron-oxide-based contrast agent serving as the reference standard. Tumor and normal-appearing white matter (NAWM) regions of interest (ROIs) are compared across injections and with and without BSW correction. Agreement is assessed using concordance correlation coefficients (CCC).Results
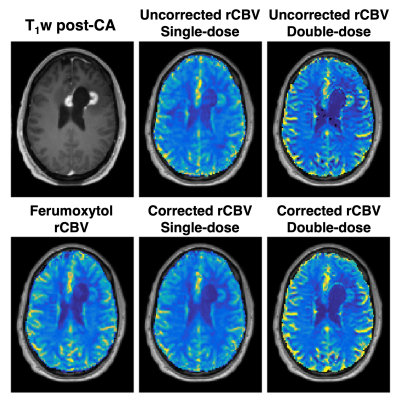
The ferumoxytol injection was well tolerated by all subjects (mean age = 58, range 46-64, 2 males). Figure 1 shows the single-dose GBCA (dual-echo no-preload), single-echo GBCA (single-echo with preload), and intravascular iron-oxide CA signals (top) and ΔR2* curves (middle) in a tumor ROI from one subject, along with the corresponding ΔR2* curves in a NAWM ROI (bottom). T1 effects are evident in the dual-echo GBCA signals (blue) due to the lack of preload, particularly for TE1, while the single-echo GBCA signal and iron-oxide CA signal do not exhibit appreciable leakage effects due to the preload and intravascular nature, respectively. The T1 leakage effects can be effectively removed for the dual-echo ΔR2*, and similar ΔR2* curves result from each GBCA injection. While the Fe-based injection shows a higher peak ΔR2* for tumor, this is offset by the higher peak ΔR2* observed in the NAWM. This can be seen in the rCBV maps in Figure 2, which show high similarity between corrected GBCA-rCBV and Fe-rCBV maps. Figure 3 illustrates the voxel-wise correlation between the GBCA-derived rCBV (uncorrected (A) and corrected (B) for single-dose dual-echo (blue) and double-dose single-echo (red)) and the iron-oxide-derived rCBV in a single subject, with the corresponding violin plots (C). CCC improved following leakage correction (uncorrected = 0.4 and 0.24; corrected = 0.70 and 0.51 for single- and double-dose, respectively). The mean rCBV values for all subjects across the three injections are shown in Table 1. Finally, Figure 4 shows group-wise correlations for mean GBCA-rCBV versus Fe-rCBV for all subjects.Discussion
Iron-oxide-based contrast agents are not affected by CA leakage effects over the dynamic time-course of DSC-MRI, and therefore this method represents a standard by which to compare rCBV accuracy. GBCA-based rCBV are often performed with double-bolus strategies to reduce the effects of CA leakage, though strategies to minimize or remove leakage effects have shown promise for single-bolus strategies (9,10). For single-bolus rCBV, T1 leakage effects are removed from the dual-echo rCBV, but remaining T2* leakage effects increase rCBV without BSW correction. Similarly, BSW correction provides more accurate rCBV for the double-bolus strategy. Overall, these results suggest that leakage-correction of GBCA-derived DSC data with the widely used BSW method produces accurate rCBV maps, which holds for both single-bolus dual-echo and double-bolus standard rCBV. Work is ongoing to compare more leakage correction methods (8) and to acquire this data in more patients.Conclusions
To date, the accuracy of leakage correction strategies for GBCA-based DSC-MRI has not been studied in human populations in conjunction with a reference standard. In this study, we showed that GBCA-derived rCBV maps are similar on a voxel-wise level to rCBV maps from iron-oxide-based reference standard using leakage correction. Ultimately, this study may improve confidence in the accuracy of DSC-derived rCBV maps acquired with GBCA and lead to more wide-scale implementation of standardized methods for DSC-MRI, which has clinical implications for patients with brain tumors.Acknowledgements
This work was supported by NIH/NCI R01 CA213158-01, the Arizona Biomedical Research Commission (ADHS16-162414), and Philips Healthcare. The authors thank Lori Steffes and Brittany Maykowski for help with patient recruitment.References
1. Boxerman JL, Schmainda KM, Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol. 2006;27(4):859–67.
2. Hu LS, Baxter LC, Smith KA, Feuerstein BG, Karis JP, Eschbacher JM, et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusio. AJNR Am J Neuroradiol. 2009;30(3):552–8.
3. Schmainda KM, Prah M, Connelly J, Rand SD, Hoffman RG, Mueller W, et al. Dynamic-susceptibility contrast agent MRI measures of relative cerebral blood volume predict response to bevacizumab in recurrent high-grade glioma. Neuro Oncol. 2014;16(6):880–8.
4. Boxerman JL, Prah DE, Paulson ES, Machan JT, Bedekar D, Schmainda KM. The Role of preload and leakage correction in gadolinium-based cerebral blood volume estimation determined by comparison with MION as a criterion standard. AJNR Am J Neuroradiol. 2012;33(6):1081–7.
5. Bjornerud A, Sorensen AG, Mouridsen K, Emblem KE. T1- and T2*-dominant extravasation correction in DSC-MRI: part I--theoretical considerations and implications for assessment of tumor hemodynamic properties. J Cereb Blood Flow Metab. 2011;31(10):2041–53.
6. Leu K, Boxerman JL, Cloughesy TF, Lai A, Nghiemphu PL, Liau LM, et al. Improved Leakage Correction for Single-Echo Dynamic Susceptibility Contrast Perfusion MRI Estimates of Relative Cerebral Blood Volume in High-Grade Gliomas by Accounting for Bidirectional Contrast Agent Exchange. Am J Neuroradiol. 2016 Aug 1;37(8):1440 LP – 1446.
7. Hu LS, Baxter LC, Pinnaduwage DS, Paine TL, Karis JP, Feuerstein BG, et al. Optimized preload leakage-correction methods to improve the diagnostic accuracy of dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging in posttreatment gliomas. Am J Neuroradiol. 2010;31(1):40–8.
8. Stokes AM, Semmineh N, Quarles CC. Validation of a T1 and T2* leakage correction method based on multiecho dynamic susceptibility contrast MRI using MION as a reference standard. Magn Reson Med. 2016;76(2):613–25.
9. Schmainda KM, Prah MA, Hu LS, Quarles CC, Semmineh N, Rand SD, et al. Moving Toward a Consensus DSC-MRI Protocol : Validation of a Low – Flip Angle Single-Dose Option as a Reference Standard for Brain Tumors. AJNR Am J Neuroradiol. 2019;40(4):626–33.
10. Nespodzany A, Alhilali LM, Hu LS, Baxter LC, Quarles CC, Stokes AM. Evaluation of Single Bolus, Multi-Echo Dynamic Susceptibility Contrast Protocols in Brain Tumor Patients. In: Proceedings of the 27th Annual Meeting of ISMRM. 2019. p. 0394.
Figures