1067
GABA and Glu in posterior cingulate cortex of mild TBI patients: MEGA-PRESS and TE averaged PRESS study with spectral averaging1Radiology, Clinical and Research Institute of Emergency Pediatric Surgery and Trauma, Moscow, Russian Federation, 2Emanuel Institute of Biochemical Physics of the Russian Academy of Sciences, Moscow, Russian Federation, 3Moscow State University, Moscow, Russian Federation, 4Philips Healthcare, Russia, Moscow, Russian Federation, 5Semenov Institute of Chemical Physics of the Russian Academy of Sciences, Moscow, Russian Federation
Synopsis
In this study, GABA and Glu are measured in the posterior cingulate cortex (PCC) of children with acute mTBI using MEGA-PRESS and TE averaged PRESS. Spectra were processed individually and after between-subject group averaging. The results demonstrate the increase in Glu without glutamine contribution and the stability of GABA without macromolecule contamination which means that the excitatory-inhibitory neurotransmitter balance is shifted towards excitation in the cerebral region studied. Spectral averaging over groups of participants recommends itself as a powerful tool, if used with proper preprocessing steps.
Introduction
To date, the mechanisms of post-traumatic disorders in mTBI are studied poorly and require additional research. Clinical and experimental data demonstrate that the row of molecular and cellular processes following mTBI (e.g. metabolism deviations or inflammation) are found in subacute post-traumatic period (from 2 weeks to 3 months after injury) [new 1]. The balance between major neurotransmitters (excitatory – glutamate (Glu), and inhibitory – γ-aminobutyric acid, GABA), is crucial for the normal functioning of CNS.In this study, MEGA-PRESS and TE averaged PRESS (TEavg) were used in order to measure [GABA] without macromolecular contamination and [Glu] without glutamine contribution in the posterior cingulate cortex (PCC) of children with acute mTBI. To increase the accuracy of measurements, spectra of all subjects in groups were averaged.
Materials & Methods:
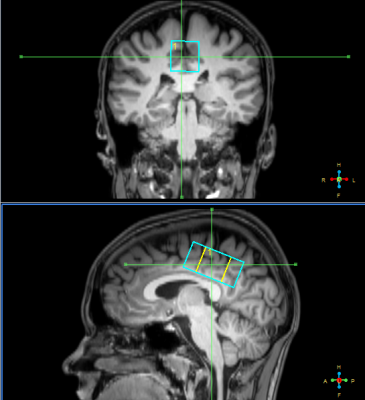
Eighteen patients with acute mTBI (up to 72 hours since the injury, aged 16.2±1.9, 8f+10m) and fifteen healthy age-matched controls (9f+6m) participated in the study. MRI scanner Philips Achieva dStream 3.0T was used with receive SENSE head coil. Standard MRI protocol for TBI patients (T2-, T1-weighted images, FLAIR, SWI, DTI) revealed no pathological lesions in brain of any subject. MR spectroscopy voxels sized 40x20x20 mm were located in the area of posterior cingulate cortex (see figure 1). To acquire GABA- signal, MEGA-PRESS with macromolecular signal suppression was used [2]: TR=2000 ms, TE=80 ms, 20 ms editing pulses on δOn=1.9 ppm, δOff=1.5 ppm, NSA=288, acquisition time ~10 min.To measure pure Glu signal, TEavg spectrum [3] was acquired: TR = 2000 ms, TE starting from 35 ms up to 185 ms with the 2.5 ms step, NSA for each TE=4, (acquisition time ~4.5 min). Unsuppressed water spectrum (TE=28 ms, TR=10000 ms) was also acquired. The voxels superimposed on 3D T1 images were segmented in spm12 [4], grey/white/CSF fractions were defined. These values together with the intensity of reference water spectrum were used in order to calculate absolute concentrations.
Spectra were processed in two ways: individually for each subject and after averaging over normal and mTBI groups. The preprocessing steps were the rejection of motion-corrupted dynamics, spectral aligning, zero-filling, phasing and averaging of the dynamics in each spectrum of each subject. Frequency and phase aligning between spectra of different subjects was performed prior to spectral averaging over groups.
Individual GABA spectra were processed in Gannet 3.0 [5], TE averaged PRESS – in LCModel. The values of GABA/Cr, Glx/Cr, [GABA] and Glu/Cr passed the normality test, after that t-test or Mann-Whitney criterion were applied to define the significance of between-group differences.
Group-averaged spectra were processed in Matlab. GABA, Cr and Glu signal intensities were calculated by GannetFit algorithm with Gaussian shape for GABA and Lorentzian – for Glu and Cr.
Results
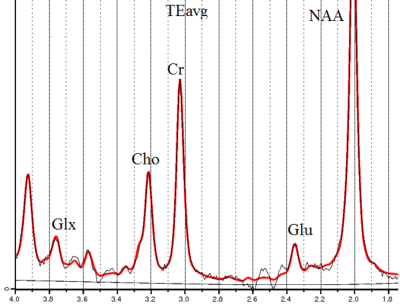
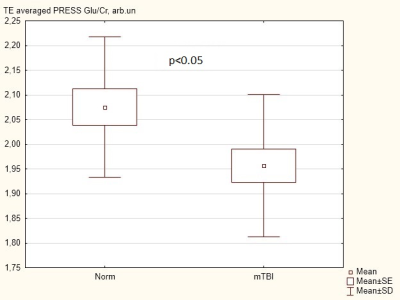
The average fit error of individually processed GABA signals was ~13%. The values of [GABA] were 1.88±0.27 mM in the norm and 1.94±0.32 mM in mTBI with no statistically significant difference. GABA/Cr, GABA/Glx and Glx/Cr values also remained unchanged.In individual TEavg spectra the approximate CRLB of Glu and Cr were ~6% and ~2%, respectively (spectral processing: figure 2). The Glu/Cr value in mTBI group decreased by 6% (p<0.05), figure 3. The concentrations of other metabolites, as well as their /Cr intensities, were unchanged.
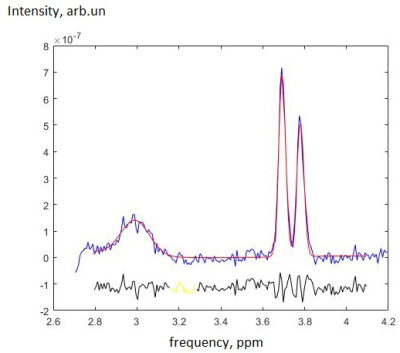
In group-averaged MEGA-PRESS spectra (see fig. 4), the fit error was ~8% for GABA signal and ~0.8% for Cr signal. The GABA/Cr values were equal: 0.094±0.008 in the norm and 0.090±0.008 in mTBI. Glx/Cr was 0.191±0.005 for norm, and 0.190±0.004 for mTBI.
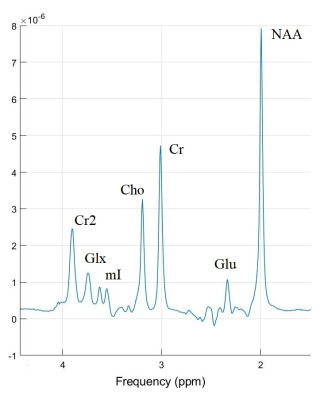
The TEavg spectrum averaged over normal group is demonstrated in fig. 5. The Glu and Cr fit errors in group-averaged TEavg spectra both were ~0.6%. The Glu/Cr values were equal to 0.161±0.004 in the norm and 0.172±0.004 in mTBI, meaning +7% difference with ~1% error.
Discussion
To our knowledge, the TE averaged PRESS was applied for Glu measurement in the acute mTBI for the first time. The result of the individual TEavg processing (and statistical analysis) disagrees with the group-averaged spectral processing. We suppose that since in averaged spectra the SNR of Glu signal is significantly higher (see fig. 4) and Glu fit error is significantly lower than its CRLB in individual spectra, the revealed in non-averaged spectra decrease in Glu/Cr with p<0.05 is false-positive, and, in fact, Glu/Cr increases. This draws attention to the data quality and indicates that the statistical significance of the effect even in case of relatively low CRLB and a sufficient number of participants may not be enough for the confident analysis. Spectral averaging over groups of participants recommends itself as a powerful tool, if used with proper preprocessing steps.So, the results demonstrate the increase in pure Glu without glutamine contribution and the stability of GABA without macromolecules in PCC of children with acute mTBI. In our previous study we have demonstrated that [GABA] increases in anterior cingulate cortex (ACC) while Glx remains unchanged [6]. The results of current study indicate that PCC reacts to the mTBI in the opposite way, and not like the ACC. Here, the excitatory-inhibitory neurotransmitter balance is shifted towards excitation. Long-term studies are of interest in order to connect the mTBI outcome with the [GABA] and [Glu] dynamics in various cerebral regions.
Acknowledgements
No acknowledgement found.References
[1] 10.3233/PRM-150347
[2] 10.1002/mrm.24391
[3] 10.1002/mrm.20007
[4] https://www.fil.ion.ucl.ac.uk/spm/doc/manual.pdf
[5] 10.1002/jmri.24478
[6] 10.1134/S0006350917060161
Figures