1061
Radio-chemotherapy induced toxic leukoencephalopathy: ultra-high field MR findings1Laboratory of Medical Physics and Magnetic Resonance, IRCCS Fondazione Stella Maris, Pisa, Italy, 2Imago7 Research Foundation, Pisa, Italy, 3Department of Developmental Neuroscience, IRCCS Fondazione Stella Maris, Pisa, Italy, 4Neuroradiology Unit, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy, 5Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy, 6Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy, 7Italian DEvelopmental Age Health Network (IDEA Network), Rome, Italy
Synopsis
The affirmation of the role of UHF MRI in the clinical setting requires the demonstration of its diagnostic and prognostic power, especially in the context of fields not yet sufficiently explored, such as diseases of pediatric age. This study presents a single case of radio-chemotherapy induced leukoencephalopathy in which UHF-MRI demonstrated its added value compared to traditional MR imaging in the characterization of lesions and in the detailed delineation of their localization.
Introduction
Recent studies have demonstrated that the increase of spatial resolution and SNR of ultra-high field (UHF) MRI has important clinical implications in the diagnosis, treatment, and follow-up of numerous diseases (such as multiple sclerosis, cerebrovascular diseases, brain tumors, epilepsy, neurodegeneration 1-4). However, further studies are necessary to evaluate the diagnostic and prognostic power of 7 Tesla in the pediatric field, especially in the context of diseases not yet sufficiently explored, such as leukoencephalopathy. Here we present the case of a 9-years old child with a radio-chemotherapy induced toxic leukoencephalopathy, studied at 7 Tesla.Background
The rare yet severe consequences of brain radiation and/or chemotherapy in survivors of childhood leukemia such as focal necrosis, necrotizing and mineralizing leukoencephalopathy, major arterial occlusion, mineralizing microangiopathy small vascular malformations, intraparenchymal cysts, and secondary neoplasms are well described 5. Here we present the case of a 9-years old child who received a diagnosis of acute lymphoid leukemia at the age of 5 years and underwent chemotherapy (both intravenous and intrathecal) and radiotherapy. After the first cycle of chemotherapy, the child underwent a first brain MR exam that was negative. Nine months after the diagnosis, the child started brain prophylaxis radiotherapy, which was wrongly executed at a triple dose (36 Gy instead of 12 Gy). A second brain MR exam, repeated after 3 years from the first (at 8 years), showed diffuse leukoencephalopathy, affecting bilaterally the deep and fronto-temporo-parietal subcortical white matter, with cystic formations.Methods
About 4 years after the diagnosis the child arrived at our Institute for a complete neuropsychiatric assessment. He performed a 1.5 T (GE HDxt, with 8 channels receiver coil) brain MRI exam including the following sequences: 3D T1-weighted (BRAVO) 1mm isotropic, 2D FSE T2-weighted, 2D SE T1-weighted, 3D FLAIR, 3D Gradient-Recalled Multi-Echo sequence (SWAN), DTI (30 directions, b-value=1000 s/mm2). After 3 days, the child underwent also ultra-high field brain MRI (Discovery MR950 7 T whole-body MRI system, GE Healthcare, equipped with a 2-channel transmit and 32-channels receive coil, Nova Medical, Wilmington, MA, USA).The high-resolution protocol included: a 3D T1-weighted (BRAVO) sequence (TE/TR=2.3ms/5ms, TI=600ms, FA=7°, FOV=220x220 mm2, matrix=276x276, slice thickness=0.8mm, ASSET acceleration=2), a 2D FSE T2-weighted sequence (TE/TR=75.8ms/7000ms, FA=90°, ETL=16, FOV=200x200 mm2, matrix=512x512, slice thickness=4mm), a 3D FLAIR sequence (TE/TR=222ms/8000ms, TI=2050ms, FA=90°, ETL=240 FOV=200x200 mm2, matrix=288x288, slice thickness=0.7mm, ASSET acceleration=2), and a 3D Gradient-Recalled Multi-Echo sequence (SWAN, 5 echoes, TR=54.1ms, TE=7.14/18.88/24.62/33.36/42.10 ms, FA=15°, FOV=180x180 mm2, matrix=512x512, slice thickness=0.7mm, ASSET acceleration=2, flow compensation).For the SWAN sequence, both the real and imaginary parts of the images obtained at each echo were saved and converted into phase and magnitude data for Quantitative Susceptibility Mapping (QSM). Maps were obtained according to 6, by using FSL7, MATLAB software (Mathworks, Natick, MA, USA), and STI suite (MATLAB code, available from UC Berkeley, USA, https://people.eecs.berkeley.edu/~chunlei.liu/software.html).
The raw phase images of individual echoes were unwrapped using a Laplacian-based algorithm 8-9 and brain masks were used for the removal of the background field via V-SHARP 10. The iLSQR 8 method was applied to the processed phase of each echo separately to compute susceptibility maps. One average QSM was obtained averaging the maps obtained for each echo 11.
Results
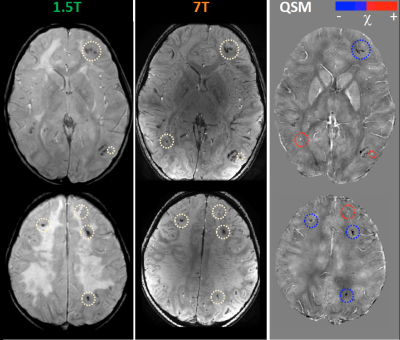
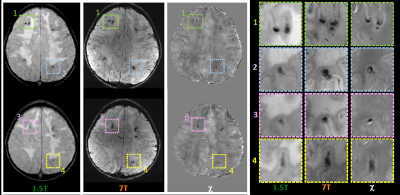
Clinical evaluations revealed mild impairment of executive functions, without specific neurological signs. Our MR images collected at 1.5T confirmed previous MR data, within the framework of leukoencephalopathy with several homogenous signal alterations apparently localized within the white matter, mainly at the cortico-medullary junction. T2-weighted images acquired at 7T showed diffuse hyper-intense signal in deep and subcortical white matter on both hemispheres. At ultra-high field MR, QSM was able to distinguish vascular malformations (small roundish paramagnetic formations due to radio-induced cavernomas or capillary telangiectasias) separately from mineralizing microangiopathy (multiple linear diamagnetic formations with a gyriform pattern referable to calcium deposition) (Figure1). The high spatial resolution of 7T images and of susceptibility maps allowed the verification that both paramagnetic and diamagnetic formations were located within the cortex, with better anatomical detail with respect to lower field images in which the lesions appeared mainly at the cortical-subcortical junction (Figure 2).Discussion and Conclusions
QSM allowed distinguishing radio-induced vascular malformations from mineralizing microangiopathy (complication dependent on combined chemo-radio treatment), classically described as affecting the basal ganglia (perforating vessels) and the cortico-medullary junction (arciform fibers). Actually, 7T MR proves that most lesions have an intracortical distribution. These data demonstrate the added value of ultra-high field MR (³7T) in the characterization of brain alterations of both white and grey matter, opening new perspectives in the study of leukoencephalopathies.Acknowledgements
This study was funding by the Italian Ministry of Health BIANCA -IDEA (RRC-2018 -2019). * Italian DEvelopmental Age Health Network (IDEA Network) is constituted by the following centers: IRCCS Ospedale pediatrico Bambino Gesù (Rome); Fondazione IRCCS Istituto neurologico “Carlo Besta” (Milan); IRCCS Ospedale pediatrico “Giannina Gaslini” (Genoa); IRCCS Eugenio Medea (Bosisio Parini); IRCCS Associazione Oasi Maria SS Onlus – Troina (Enna); IRCCS Burlo Garofalo (Trieste); Fondazione IRCCS Istituto Neurologico “Casimiro Mondino” (Pavia); IRCCS Ospedale San Raffaele (Milan); IRCCS Fondazione Stella Maris (Pisa).References
1.Trattnig S, Springer E, Bogner W, et al. Key clinical benefits of neuroimaging at 7T. Neuroimage. 2018; 168:477-489.
2.Sinnecker T, Mittelstaedt P, Dörr J, et al. Multiple sclerosis lesions and irreversible brain tissue damage: a comparative ultrahigh-field strength magnetic resonance imaging study.Arch Neurol. 2012;69(6):739-45.
3. Feldman RE, Delman BN, Pawha PS, et al. 7T MRI in epilepsy patients with previously normal clinical MRI exams compared against healthy controls. PLoS One. 2019 Mar 19;14(3):e0213642.
4. Cosottini M. et al. MR Imaging of the Substantia Nigra at 7T Enables Diagnosis of Parkinson Disease. Radiology, 271:831-8,2014.
5. Sabin ND, Cheung YT, Reddick WE, et al., The Impact of Persistent Leukoencephalopathy on Brain White Matter Microstructure in Long-Term Survivors of Acute Lymphoblastic Leukemia Treated with Chemotherapy Only. AJNR Am J Neuroradiol. 2018 Oct; 39(10): 1919–1925.
6. Lancione M, Donatelli G., Cecchi P., et al. Echo-time dependency of quantitative susceptibility mapping reproducibility at different magnetic field strengths. NeuroImage 197 (2019) 557–564
7. Smith, S.M., 2002. Fast robust automated brain extraction. Hum. Brain Mapp. 17,143–155
8. Li, W., Wu, B., Liu, C., 2011. Quantitative susceptibility mapping of human brain reflects spatial variation in tissue composition. Neuroimage 55, 1645–1656.
9. Schofield, M. a, Zhu, Y., 2003. Fast phase unwrapping algorithm for interferometric applications. Opt. Lett. 28, 1194–1196.
10. Schweser, F., Deistung, A., Lehr, B.W., Reichenbach, J.R., 2011. Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: an approach to in vivo brain iron metabolism? Neuroimage 54, 2789–2807.
11. Denk, C., Rauscher, A., 2010. Susceptibility weighted imaging with multiple echoes. J. Magn. Reson. Imaging 31, 185–191
Figures