1060
White Matter Microstructural Alterations In Contact-Sport Athletes With And Without Concussion: A Multi-Shell Diffusion Study
Sohae Chung1,2, Junbo Chen3, Tianhao Li3, Yao Wang3, and Yvonne W. Lui1,2
1Center for Advanced Imaging Innovation and Research (CAI2R), Department of Radiology, New York University Grossman School of Medicine, New York, NY, United States, 2Bernard and Irene Schwartz Center for Biomedical Imaging, New York University Grossman School of Medicine, New York, NY, United States, 3Department of Electrical and Computer Engineering, NYU Tandon School of Engineering, Brooklyn, NY, United States
1Center for Advanced Imaging Innovation and Research (CAI2R), Department of Radiology, New York University Grossman School of Medicine, New York, NY, United States, 2Bernard and Irene Schwartz Center for Biomedical Imaging, New York University Grossman School of Medicine, New York, NY, United States, 3Department of Electrical and Computer Engineering, NYU Tandon School of Engineering, Brooklyn, NY, United States
Synopsis
There is growing concern that there may be negative effects on the brain from repetitive head impacts as well as sport-related concussion (SRC). Here, we demonstrate widespread white matter microstructural differences between contact-sport athletes and non-contact sport controls using multi-shell diffusion MRI. Importantly, microstructure differences were present in contact-sport athletes both with and without concussion, suggesting that exposure to multiple head impacts effects brain microstructure. Decreased radial diffusivity (RD) and extra-axonal radial diffusivity (D$$$e,\perp$$$) and increased fractional anisotropy (FA) suggest pathologies such as cytotoxic edema may be present acutely after injury.
INTRODUCTION
Sport-related concussion (SRC) is a significant health problem particularly among young athletes.1 There is growing concern that repetitive head impacts and concussion in contact-sport athletes may predispose to long-term consequences including risk of neurodegenerative disorders such as chronic traumatic encephalopathy (CTE).2 Unfortunately, our understanding of the underlying mechanisms of injury and pathophysiology associated with SRC is limited. Here we investigate white matter (WM) microstructure in three groups: contact-sport athletes with (CS-SRC) and without SRC (CS-nSRC) and non-contact athlete controls (NCS-Control), and we use compartment specific WM tract integrity (WMTI) metrics3 derived from multi-shell diffusion MRI as well as diffusion tensor/kurtosis imaging (DTI/DKI) metrics to characterize WM microstructure between groups.METHODS
Data including MRI were used from datasets available through the Federal Interagency Traumatic Brain Injury Research (FITBIR) registry from the NCAA-DoD CARE Consortium.4 Among available datasets, we included only those data which satisfied the following criteria: 1) available multi-shell diffusion MR images, 2) performed on 3T Prisma scanners (Siemens), 3) male subjects, 4) imaging performed within 24-48 hours of injury. 34 contact-sport athletes: 16 CS-SRC (19.6 ± 0.9 years old), 18 CS-nSRC (19.7 ± 1.5 years old), and 25 NCS-Control (19.6 ± 1.1 years old) were included. Multi-shell diffusion imaging with the following acquisition parameters were analyzed: 2 b-values (1000, 2000 s/mm2), 30 diffusion directions, 8 b0 images, TE/TR = 98/7900 ms, FOV = 243 mm, matrix = 90x90, 64 slices, 2.7 mm isotropic.WMTI maps were calculated (axonal water fraction [AWF], intra-axonal diffusivity [Daxon], extra-axonal axial and radial diffusivities [De,|| and De,$$$\perp$$$]), as well as DTI metrics (fractional anisotropy [FA], mean, axial, radial diffusion coefficients [MD, AD, RD]) and DKI metrics (mean, axial, radial kurtosis [MK, AK, RK]). Tract-based spatial statistics (TBSS)5 were performed to test between-group differences. Resulting statistical maps were thresholded at p < 0.05 (corrected for multiple comparisons).
RESULTS
Fig.1 shows the spatial distribution of the TBSS results for significantly differences voxels between groups. Widespread areas of decreased MD, RD and De,$$$\perp$$$, increased FA and AK were observed in the CS-SRC group compared to NCS-Controls, including in major WM tracts such as corpus callosum, external/internal capsules, corona radiata, cerebral peduncle, posterior thalamic radiation, superior longitudinal fasciculus (Fig. 1, top). Similar results were also found between NCS-Control and CS-nSRC groups, showing significantly decreased MD, RD and increased FA, MK, AK, RK, AWF, Daxon, De,|| (Fig. 1, bottom). No differences were found between CS-nSRC and CS-SRC groups.DISCUSSION
Here we demonstrate that diffusion microstructure metrics can detect widespread WM changes after SRC. We mainly observed decreases in RD and De,$$$\perp$$$ and increased FA in CS-SRC subjects acutely after injury (Fig.1, top). As suggested by previous work6, cytotoxic edema is a possible mechanism which could lead to restrictions to diffusion in the extra-axonal space, particularly in the perpendicular direction along the axon resulting in these diffusion results. We also found higher AK in the CS-SRC group, mainly in the posterior thalamic radiation and splenium of the corpus callosum. High kurtosis reflects diffusional heterogeneity also in keeping with cytotoxic edema, observed previously in cerebral ischemia.7In contrast to a previous report8, we did find significant differences between NCS-Control and CS-nSRC groups (Fig.1, bottom). In particular, we observe increased kurtosis, AWF, Daxon and De,|| not reported before. It is not clear what physiologic factors may be driving these differences; however, it is notable that microstructure differences are present widely in the white matter between control subjects and even those contact sport athletes without concussion, suggesting that exposure to multiple head impacts itself, without discrete concussion, effects the white matter microstructure. Some of these findings are new compared with what has been previously published using these data. Here, we studied compartment modeled white matter metrics and also restricted the analysis to single acquisition device type in order to gain more homogeneity in the data.
CONCLUSION
The findings highlight effects of head impacts in contact-sport athlete both with and without SRC and provide potentially useful noninvasive imaging biomarkers for sports-related brain microstructural injury.Acknowledgements
This work was supported in part by NIH NNDS R01 NS039135, R21 NS090349, R56 NS119767, DoD PT190013, Lowenstein Foundation.References
- JA Langlos et al. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375-8.
- Committee on Sports-Related Concussions in Youth et al. Chapter 5. Consequences of repetitive head impacts and multiple concussions. In: R Graham et al, editors. Sports-related concussions in youth: Improving the science, changing the culture. Washington DC: National Academies Press; 2014. (http://www.careconsortium.net)
- E Fieremans et al. White matter characterization with diffusion kurtosis imaging. Neuroimage. 2011;58:177-188.
- SP Broglio et al. A national study on the effects of concussion in collegiate athletes and US military service academy members: the NCAA-DoD concussion assessment, research and education (CARE) consortium structure and methods. Sports Med. 2017;47:1437-1451.
- SM Smith et al. Track-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487-1505.
- Lin et al. Simulation of changes in diffusion related to different pathologies at cellular level after traumatic brain injury. MRM. 2017;76:290-300.
- JH Jensen et al. Preliminary observation of increased diffusional kurtosis in human brain following recent cerebral infarction. NMR Biomed. 2010;24:452-457.
- YC Wu et al. Longitudinal white-matter abnormalities in sports-related concussion: a diffusion MRI study. Neurology. 2020;95:e781-e792.
Figures
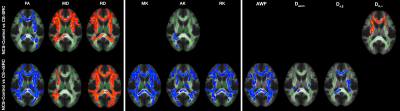
Figure 1: TBSS results showing comparisons between three groups
for DTI, DKI, WMTI metrics. Clusters of significantly decreased/increased
voxels in (top) CS-SRC and (bottom)
CS-nSRC (p < 0.05) are shown in red/blue, respectively, and overlaid on the
standard template, together with the mean FA skeleton (green). No significant
differences were found between CS-nSRC and CS-SRC groups. Also, AD was not
significant in all comparisons.