1058
Hippocampal and Anterior Cingulate Blood Flow is Associated with Affective Symptoms in Chronic Traumatic Brain Injury1Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 2Department of Bioengineering, University of Texas at Arlington, Arlington, TX, United States, 3Department of Neurology, University of Texas Southwestern Medical Center, Dallas, TX, United States, 4Institute for Exercise and Environmental Medicine, Texas Health Presbyterian Hospital, Dallas, TX, United States, 5Department of Radiology and Cognitive Imaging Research Center, Michigan State University, East Lansing, MI, United States, 6Department of Psychiatry, University of Texas Southwestern Medical Center, Dallas, TX, United States, 7Department of Neurological Surgery, University of Texas Southwestern Medical Center, Dallas, TX, United States, 8Department of Neurology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, United States, 9Department of Physical Medicine and Rehabilitation, University of Texas Southwestern Medical Center, Dallas, TX, United States
Synopsis
Chronic Traumatic-brain-injury (TBI) has lifelong implications on brain function. It is characterized by cerebral-blood-flow (CBF) deficits, often accompanied by TBI-related symptoms. It is crucial that we understand mechanisms of CBF alterations and its association with TBI-symptoms. We observed CBF deficits in patients with TBI in the thalamus, hippocampus and other subcortical structures compared to a group of normal control participants. Furthermore, CBF in the hippocampus and anterior cingulate were negatively associated with TBI-related symptoms of anxiety, depression, fatigue and sleep issues. Our results suggest that regional CBF deficits may be useful biomarkers for perfusion-targeted therapies to ameliorate TBI-related symptoms.
Purpose
The purpose of this study was to assess cerebral blood flow (CBF) in patients with chronic traumatic brain injury (TBI) compared to normal control participants and evaluate the association of CBF with self-reported TBI-symptoms.Introduction
TBI is a chronic condition with lifelong implications on brain function. Along with neuronal and axonal injury, cerebrovascular dysfunction is well documented acutely after TBI1. Vascular injuries may recover over time, but CBF deficits are observed long term due to incomplete recovery2-5. These CBF deficits may lead to deterioration of brain function and related symptoms like anxiety, anger, depression, fatigue and sleep disorders, which lead to poor quality of life. It is crucial that we understand mechanisms of CBF alterations and its association with TBI-symptoms to plan future treatments.Methods
16 patients with chronic (average 20 months after injury) TBI (11 mild, 5 moderate/severe) were recruited to this HIPAA compliant study, approved by IRBs at UT Southwestern, Parkland, and Presbyterian Hospitals, and performed in accordance with guidelines of the Declaration of Helsinki and Belmont Report. All participants granted informed consent. Information about patient characteristics, cognitive function and symptom scores are reported in Table1. Cognitive and emotional functions were assessed with the NIH Toolbox Cognitive Battery and the Patient-Reported-Outcome-Measurement-Information-System (PROMIS) which generated T-scores adjusted for age, sex, education, and race/ethnicity. Cognitive scores one standard deviation (SD) below mean T-scores for the general population were considered abnormal6.3D magnetization-prepared-rapid-acquisition-of-gradient-echo (MPRAGE), and 2D pseudo-continuous ASL (PCASL) data were acquired on a 3-Telsa scanner (Philips) with an 8-channel head coil. MPRAGE data with 1×1×1mm3 resolution was acquired in 4-mins. ASL data were acquired at rest using a 6-min 2D-PCASL single-shot echo-planar-imaging (EPI) sequence7, with following parameters: label duration=1650ms, post-label delay=1525ms, TR/TE=4260/14ms, voxel size=3x3x5mm3, field-of-view (FOV)=240x240mm, flip angle=90°, 29 slices, 40 label/control pairs, no background suppression.
For comparison, a group of normal control (NC) participants with matching age and sex as the patients were randomly selected from our prior study. Cognitive function was not assessed in the NC participants.
Results, discussion and conclusion
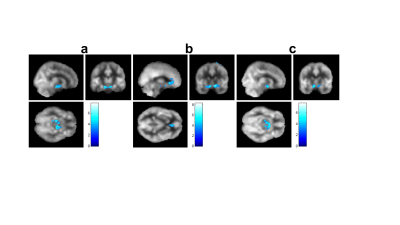
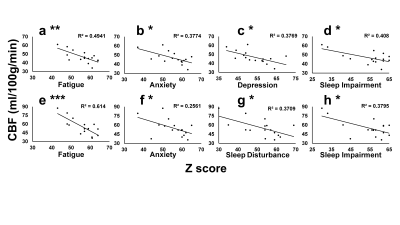
Group comparison of whole brain voxel-wise CBF maps revealed significantly lower CBF bilaterally in the thalamus, left hippocampus and parahippocampus in patients with TBI (Figure 1). Consistent with the voxel-wise analysis, region of interest (ROI) analysis showed lower CBF bilaterally in the thalamus and hippocampus, left caudate and amygdala in patients with TBI (Table 2). All mean cognitive function and PROMIS scores were within the average range, suggesting relatively good recovery of most patients with TBI. A negative correlation was observed between voxel-wise CBF and symptoms of anger, anxiety and depression after controlling for age and sex (Figure2). Patients with TBI who reported greater anger symptoms had lower CBF in the hippocampus, parahippocampus, and lentiform nucleus (Figure 2a). Patients with higher anxiety had lower CBF in the anterior cingulate (BA 32), bilateral hippocampus and parahippocampus (BA 28), and bilateral inferior frontal gyrus (BA 13) (Figure 2b). Patients with severe depression symptoms had lower CBF in the hippocampus, parahippocampus and amygdala (Figure 2c). Higher anger, anxiety and depression scores were commonly associated with lower hippocampal CBF despite mean PROMIS scores in the normal range. Negative correlations (Pearson) were observed between hippocampus ROI CBF and symptoms (Figure 3 a - d) of Fatigue (p=0.002), Anxiety (p=0.01), Depression (p=0.01), and Sleep impairment (p=0.008). Negative correlations were also observed between rostral anterior cingulate CBF and symptom scores (Figure 3 e - h) of Fatigue (p=0.0003), Anxiety (p=0.045), Sleep Disturbance (p=0.01), and Sleep impairment (p=0.01).This study demonstrated that 1) patients with TBI with ongoing neurological symptoms had significantly lower CBF bilaterally in the thalamus, hippocampus, parahippocampus, the left caudate and amygdala despite relatively intact brain volume and cognitive function. 2) Voxel-wise regression revealed that CBF deficits, mostly in the hippocampus and rostral anterior cingulate were associated with higher levels of self-reported symptoms of anger, anxiety, and depression; notably CBF in the hippocampus and rostral anterior cingulate was negatively correlated with most TBI-related symptoms.
Taken together, these findings provide additional evidence that regional CBF deficits exists in the chronic stage of TBI and lower CBF in the hippocampus and rostral anterior cingulate may play a role in self-reported affective symptoms. To our knowledge, this is the first study to report negative associations between regional CBF and self-reported TBI-related symptoms as measured by PROMIS. Our results are in agreement with previous reports of negative associations between CBF and other TBI-related symptoms of headache and disinhibitive behavior3,8. Of note, a causal relationship between regional CBF and TBI-symptoms cannot be addressed from this or previous cross-sectional studies3,8, and it should be noted that mean scores across cognitive and emotional measures were within normal limits, which may have limited the magnitude of the correlations. Based on prior research in humans, we suggest that microvascular injury after TBI may lead to regional CBF deficits, which is worsened in the chronic stage and is associated with affective symptoms3,8. Results also suggest that regional CBF deficits may be useful predictive and / or pharmacodynamics biomarkers for perfusion- targeted therapies to ameliorate chronic TBI-related symptoms.
Acknowledgements
We thank all participants for their time in this study and NIH for funding.References
1. Jullienne A, Obenaus A, Ichkova A, Savona-Baron C, Pearce WJ, Badaut J. Chronic cerebrovascular dysfunction after traumatic brain injury. J Neurosci Res. Jul 2016;94(7):609-22. doi:10.1002/jnr.23732
2. Kenney K, Amyot F, Haber M, et al. Cerebral Vascular Injury in Traumatic Brain Injury. Experimental neurology. Jan 2016;275 Pt 3:353-66. doi:10.1016/j.expneurol.2015.05.019
3. Gilkey SJ, Ramadan NM, Aurora TK, Welch KM. Cerebral blood flow in chronic posttraumatic headache. Headache. Oct 1997;37(9):583-7. doi:10.1046/j.1526-4610.1997.3709583.x 4. Kim J, Whyte J, Patel S, et al. Resting cerebral blood flow alterations in chronic traumatic brain injury: an arterial spin labeling perfusion FMRI study. J Neurotrauma. Aug 2010;27(8):1399-411. doi:10.1089/neu.2009.1215
5. Haber M, Amyot F, Kenney K, et al. Vascular Abnormalities within Normal Appearing Tissue in Chronic Traumatic Brain Injury. J Neurotrauma. Oct 1 2018;35(19):2250-2258. doi:10.1089/neu.2018.5684
6. Tulsky DS, Carlozzi NE, Holdnack J, et al. Using the NIH Toolbox Cognition Battery (NIHTB-CB) in individuals with traumatic brain injury. Rehabil Psychol. Nov 2017;62(4):413-424. doi:10.1037/rep0000174
7. Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. Jan 2015;73(1):102-16. doi:10.1002/mrm.25197
8. Oder W, Goldenberg G, Spatt J, Podreka I, Binder H, Deecke L. Behavioural and psychosocial sequelae of severe closed head injury and regional cerebral blood flow: a SPECT study. J Neurol Neurosurg Psychiatry. Jun 1992;55(6):475-80. doi:10.1136/jnnp.55.6.475
Figures
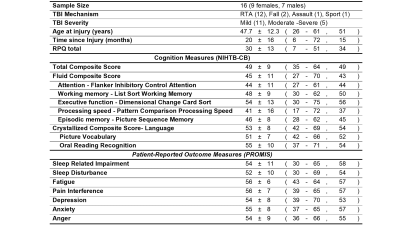
Table 1. TBI information, cognitive and symptom scores
All data are presented in mean ± standard deviation (minimum – maximum, median). RTA: road traffic accidents which include motor vehicle accident, motorcycle collision, and motor vehicle pedestrian accident. RPQ: Rivermead Post-Concussion Symptoms Questionnaire. Cognition measures and PROMIS data are presented as normalized T-scores (mean of 50, SD = 10) corrected for age, sex, education, and race/ethnicity in the general population.