1057
Investigating the extent of difference in single tensor and beyond single tensor diffusion MRI-derived voxelwise measures1Cleveland Clinic Lou Ruvo Center for Brain Health, Las Vegas, NV, United States, 2University of Washington - Seattle, Seattle, WA, United States
Synopsis
In this study, we investigated the spatial extent and location of white-matter (WM) disorganization due to repeated head impacts (RHI) by estimating single-tensor (ST) diffusion MRI (dMRI)-measures along with free-water (FW)-corrected ST measures, diffusion kurtosis imaging (DKI), and Neurite Orientation Dispersion and Density Imaging (NODDI) measures, along with understanding the correlation of such voxelwise measures with exposure to fighting and neuropsychological scores. Overall, our findings suggest that WM disorganization is prevalent in thalamocortical and corpus-callosum fibers due to RHI, although, the spatial extent and location of these differences are heavily dependent on the dMRI-models utilized in the study.
Introduction
It is well-known that single-tensor (ST) diffusion MRI (dMRI)-derived measures are biased due to crossing-fibers1, presence of free-water (FW)2, and choice of analytic techniques used3 to understand white-matter (WM) disorganization due to underlying pathology. Estimating FW-corrected ST measures along with a few widely utilized beyond ST analytic techniques such as Diffusion Kurtosis Imaging (DKI)4 and Neurite Orientation Dispersion and Density Imaging (NODDI)5 are proposed to mitigate some of the biases of ST dMRI-derived measures. However, the relative improvement of these popular techniques over ST dMRI-derived conclusions to understand WM disorganization due to pathology is still unclear, thereby restricting the widespread applicability of these techniques in routine clinical investigations. Repetitive head impact (RHI) is thought to induce robust WM damage6 which may be the risk factor for various disorders7–9. Indeed, various voxelwise ST dMRI-derived measures such as fractional anisotropy (FA) and mean diffusivity (MD) have shown differences in the temporo-occipital white matter tracts and forceps major10–17 due to RHI. Hence, in this study, we investigated the spatial extent and location of WM disorganization due to RHI by estimating ST dMRI-measures along with FW-corrected ST measures, DKI, and NODDI measures, along with understanding the correlation of such voxelwise measures with exposure to fighting and neuropsychological scores.Methods
Participants: Twelve male active professional boxers (Age: 34.33±5.53 years; Age of first professional fight: 15.17±6.44 years; Years of professional fights: 10.75±4.73; Number of professional fights: 24.67±15.59; Education: 13.17±1.99 years) and ten healthy male controls (HC; Age: 35.5±11.07 years; Education: 15±1.56 years) were recruited at our center. Neuropsychological Assessment: All participants completed neuropsychological assessments using CNS vital signs18 on a computer in a quiet room supervised by a researcher on the same visit. Four measures, namely processing speed, psychomotor speed, verbal memory, and reaction time were collected from every participant. dMRI acquisition: dMRI was acquired for all participants on a 3T Siemens Skyra using CMRR pulse sequence with 2 shells of b=1000s/mm2 and 2500s/mm2 each with 71 diffusion encoding directions (DEC), 8 non-diffusion weighted (b0) images interspersed between the DEC for each shell, Multiband factor=3, GRAPPA=2, TR=5218ms, TE=100ms, resolution=1.5mm3, and phase-encoding directions of P>>A. We also acquired an opposite phase-encoding b0 image with the same acquisition protocol. Total acquisition time was 18 minutes. Preprocessing: All data were corrected for eddy-current distortion using eddy19 tools from FSL and head motion was computed across the session for each participant. Processing: (i) ST dMRI-derived measures were estimated using dtifit tool of FSL; (ii) FW and FW-corrected ST dMRI measures were obtained using DiPY implementation of multi-shell dMRI acquisition20; (iii) DKI-derived measures were obtained using DKI MATLAB toolbox21; (iv) NODDI measures were obtained using NODDI MATLAB toolbox22. Statistical Analysis: PALM toolbox23 in FSL was used to extract significantly different or correlated ST or beyond ST dMRI-derived measures with neuropsychological scores and exposure to fighting. Significance was established at pcorr<0.05, and was family-wise error correction was performed across various measures for every technique utilized in PALM. Of note, age and education were utilized as covariates of no interest.Results
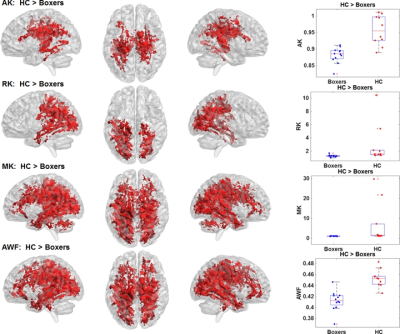
As expected, significantly lower processing speed and verbal memory were obtained in boxers as compared to HC. Average head motion along the slice encoding direction was less than 1.5mm for all participants and was not found to be significantly different between the groups (p=0.84). STFA in the thalamocortical WM tracts along with corpus callosum (CC) was significantly lower in boxers as compared to HC (Fig.1-top). These differences were observed due to an increased radial diffusivity (RD) and MD in boxers (Fig.1-middle and bottom). RD of left cingulate and MD of thalamocortical and CC was found to be negatively correlated with processing speed in HC (Fig.2-top and middle). Age of first professional fight was found to be negatively correlated with MD in boxers (Fig.2-bottom). Similarly, FWFA, FWRD, and FWMD were also observed to have the same pattern as ST dMRI-derived measures, though, the spatial extent and location were much lower than ST observation (Fig.3). DKI measures such as axonal kurtosis (AK), radial kurtosis (RK), mean kurtosis (MK), and axonal water fraction (AWF) were all observed to be significantly lower as compared to HC and were observed preferentially towards the ventral and dorsal WM regions (Fig.4). Fraction of intracellular fraction (FICVF) derived using NODDI was observed to be significantly higher in HC (Fig.5). None of FW-corrected or beyond ST dMRI-derived measures showed any correlation with exposure to fighting or neuropsychological scores in either group.Discussion and Conclusion
Our study suggests that ST dMRI-derived measures tend to show false-positive WM disorganization due to RHI, along with a false positive correlation with clinical measures. FW-correction improves the false-positivity though when compared to beyond ST measures appears to over-correct these differences. DKI and NODDI measures suggest utilization in routine clinical investigations though DKI measures are contaminated by outliers. Overall, our findings suggest that WM disorganization is prevalent in thalamocortical and CC fibers due to RHI, although, the spatial extent and location of these differences are heavily dependent on the dMRI-models utilized in the study.Acknowledgements
This study is supported by the National Institutes of Health (R01NS117547 and P20GM109025), a private grant from the Peter and Angela Dal Pezzo funds, a private grant from Lynn and William Weidner, a private grant from Stacie and Chuck Matthewson and the Keep Memory Alive Young Scientist Award at Cleveland Clinic Lou Ruvo Center for Brain Health. The Professional Fighters Brain Health Study is supported by Belator, UFC, the August Rapone Family Foundation, Top Rank, and Haymon Boxing.References
Wheeler-Kingshott CAM, Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med. John Wiley & Sons, Ltd; 2009;61:1255–1260.
2. Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn Reson Med. United States; 2009;62:717–730.
3. Mishra VR, Sreenivasan KR, Zhuang X, Yang Z, Cordes D, Walsh RR. Influence of Analytic Techniques on Comparing DTI-Derived Measurements in Early Stage Parkinson’s Disease. Heliyon. 2019;5.
4. Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. United States; 2005;53:1432–1440.
5. Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. United States; 2012;61:1000–1016.
6. Briggs DI, Angoa-Pérez M, Kuhn DM. Prolonged Repetitive Head Trauma Induces a Singular Chronic Traumatic Encephalopathy–Like Pathology in White Matter Despite Transient Behavioral Abnormalities. Am J Pathol. 2016;186:2869–2886.
7. Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Temkin N. Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil. United States; 2009;24:439–451.
8. Bigler ED. Traumatic brain injury, neuroimaging, and neurodegeneration. Front Hum Neurosci. Frontiers Media S.A.; 2013;7:395.
9. Jordan BD. The clinical spectrum of sport-related traumatic brain injury. Nat Rev Neurol. England; 2013;9:222–230.
10. Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, Lipton ML. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am J Neuroradiol. United States; 2013;34:2064–2074.
11. Mishra VR, Zhuang X, Sreenivasan KR, et al. Multimodal MR imaging signatures of cognitive impairment in active professional fighters. Radiology. 2017;285.
12. Ng TSC, Lin AP, Koerte IK, et al. Neuroimaging in repetitive brain trauma. Alzheimers Res Ther. BioMed Central; 2014;6:10.
13. Orrison WW, Hanson EH, Alamo T, et al. Traumatic brain injury: a review and high-field MRI findings in 100 unarmed combatants using a literature-based checklist approach. J Neurotrauma. 2009;26:689–701.
14. Shin W, Mahmoud SY, Sakaie K, et al. Diffusion Measures Indicate Fight Exposure-Related Damage to Cerebral White Matter in Boxers and Mixed Martial Arts Fighters. Am J Neuroradiol. 2014;35:285–290.
15. Wintermark M, Sanelli PC, Anzai Y, Tsiouris AJ, Whitlow CT. Imaging evidence and recommendations for traumatic brain injury: advanced neuro- and neurovascular imaging techniques. AJNR Am J Neuroradiol. United States; 2015;36:E1–E11.
16. Zhang L, Heier LA, Zimmerman RD, Jordan B, Ulug AM. Diffusion anisotropy changes in the brains of professional boxers. AJNR Am J Neuroradiol. 2006;27:2000–2004.
17. Zhang L, Ravdin LD, Relkin N, et al. Increased diffusion in the brain of professional boxers: a preclinical sign of traumatic brain injury? AJNR Am J Neuroradiol. 2003;24:52–57.
18. Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch Clin Neuropsychol. 2006;21:623–643.
19. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. United States; 2016;125:1063–1078.
20. Hoy AR, Ly M, Carlsson CM, et al. Microstructural white matter alterations in preclinical Alzheimer’s disease detected using free water elimination diffusion tensor imaging. PLoS One. United States; 2017;12:e0173982.
21. https://cai2r.net/resources/software/diffusion-kurtosis-imaging-matlab-toolbox.
22. https://www.nitrc.org/projects/noddi_toolbox.
23. Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. United States; 2014;92:381–397.
Figures