1056
Topological reorganization due to repetitive head impacts: Insights from Professional Fighters Brain Health Study1Cleveland Clinic Lou Ruvo Center for Brain Health, Las Vegas, NV, United States, 2University of Washington - Seattle, Seattle, WA, United States
Synopsis
Repetitive head impact (RHI) is thought to induce robust white-matter (WM) damage which may be the risk factor for various disorders. Though single-tensor diffusion MRI (dMRI) studies have improved our understanding of WM abnormalities at a regional level in participants exposed to RHI, there are no studies that attempt to understand topological WM structural connectivity changes due to RHI. Utilizing a high spatial resolution (1.5mm3) dMRI from 12 active male boxers and 10 demographically matched healthy controls (HC), we showed that RHI induces a topological shift as compared to HC, and this shift is correlated with neuropsychological scores.
Introduction
Repetitive head impact (RHI) is thought to induce robust white-matter (WM) damage1 which may be the risk factor for various disorders2–4. Indeed, various voxelwise single-tensor (ST) diffusion MRI (dMRI)-derived measures such as fractional anisotropy (FA) and mean diffusivity (MD) have shown differences in the temporo-occipital WM tracts and forceps major5–12 due to RHI. Though such studies have improved our understanding of WM abnormalities at a regional level in participants exposed to RHI, there are no studies that attempt to understand topological WM structural connectivity changes due to RHI, despite RHI being thought of as a ‘disconnection syndrome’13. Hence, in this study, we investigated the topological organization of WM-derived structural connectome due to RHI in boxers and studied the correlation between various graph-theoretical measures and exposure to fighting.Methods
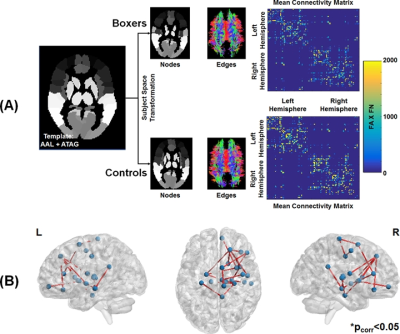
Participants: Twelve male active professional boxers (Age: 34.33±5.53 years; Age of first professional fight: 15.17±6.44 years; Years of professional fights: 10.75±4.73; Number of professional fights: 24.67±15.59; Education: 13.17±1.99 years) and ten healthy male controls (HC; Age: 35.5±11.07 years; Education: 15±1.56 years) were recruited at our center. Neuropsychological Assessment: All participants completed neuropsychological assessments using CNS vital signs14 on a computer in a quiet room supervised by a researcher on the same visit. Four measures, namely processing speed, psychomotor speed, verbal memory, and reaction time were collected from every participant. dMRI acquisition: dMRI was acquired for all participants on a 3T Siemens Skyra using CMRR pulse sequence with 2 shells of b=1000s/mm2 and 2500s/mm2 each with 71 diffusion encoding directions (DEC), 8 non-diffusion weighted (b0) images interspersed between the DEC for each shell, Multiband factor=3, GRAPPA=2, TR=5218ms, TE=100ms, resolution=1.5mm3, and phase-encoding directions of P>>A. We also acquired an opposite phase-encoding b0 image with the same acquisition protocol. Total acquisition time was 18 minutes. Preprocessing: All data were corrected for eddy-current distortion using eddy15 tools and head motion was computed across the session for each participant. Processing: Standard processing steps were used to fit diffusion tensors after eddy current distortion correction in FSL. Network construction: AAL (mainly cortical)16 and ATAG (mainly subcortical)17 atlas was used to generate 102 nodes of the network. T1-weighted MNI152 brain was normalized to each subject’s native diffusion space and the resultant transformation matrix was applied to the AAL+ATAG atlas to get the nodes in the subject’s native space. Whole-brain tractography was performed using diffusion toolkit (http://www.trackvis.org/dtk/)18. Fibers smaller than 10mm19 or having FA<0.2 were removed from any further analysis. Each internode connection (edge) was weighted by the product of the number of fibers and average FA of the fibers connecting the two nodes. Using whole-brain WM connectivity, we also computed subject scores for each participant20. Graph-theoretical measures: Various global and local graph-theoretical measures were computed using GRETNA21. Various sparsity thresholds (5-40%, step=1%) were used to identify the minimum sparsity at which the network is fully connected. A rich-club analysis was also performed to understand whether the structural connectivity pattern has preferentially organized to form distinctive network hubs in both groups. Statistical analysis: Network-based statistic (NBS)22 was used to statistically quantify differences in the weighted structural connectivity pattern between the groups. A linear regression (using PALM)23 between graph-theoretical properties and neuropsychological scores was performed to further understand the neuroanatomical correlates of the neuropsychological scores. All statistical comparisons were corrected for family-wise error at pcorr<0.05. Of note, age and education were utilized as covariates of no interest.Results
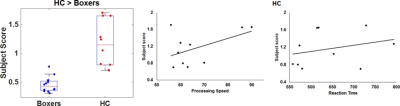
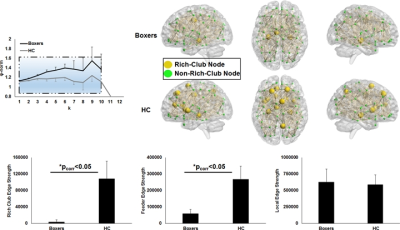
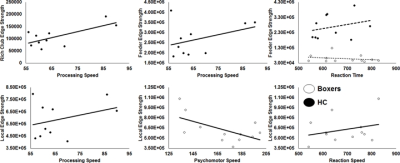
As expected, significantly lower processing speed and verbal memory were obtained in boxers as compared to HC. Average head motion along the slice encoding direction was less than 1.5mm for all participants and was not found to be significantly different between the groups (p=0.84). NBS revealed weak structural connectivity in boxers involving the thalamus, caudate, hippocampus, and frontal WM tracts (Fig.1B). Subject scores derived from whole-brain connectivity was significantly weaker in boxers (Fig.2). Subject score in HC was positively correlated with processing speed and reaction time. The network of both boxers and HC achieved a plateau of whole-brain connectivity at the sparsity threshold of 7%, although the number of nodes connected at 7% sparsity was significantly less (p=0.007) in boxers. HC showed significantly lower small-worldness at sparsity of 5% and 18%. Local efficiency of the whole network was significantly positively correlated with processing speed in HC (Fig.3). No local measures were different between the groups. Both boxers and HC showed the presence of rich-club. However, the brain regions exhibiting rich-club properties in boxers were only bilateral hippocampus and left middle cingulum while HC exhibited rich-club properties in the bilateral hippocampus, bilateral superior frontal gyrus, right supplementary motor area, bilateral insula, bilateral caudate, left putamen, and left anterior and middle cingulum (Fig.4). This observation was accompanied was significantly lower rich-club and feeder edge strength in boxers (Fig.4). A significant correlation was observed between neuropsychological scores and rich-club edge strength and feeder edge strength for both HC and boxers (Fig.5).Discussion and Conclusion
Our study shows that RHI induces a topological shift that is correlated with neuropsychological scores. Our study also suggests that there is a minimum threshold of whole-brain WM-derived structural connectivity as there was an inverted U-shape pattern that was obtained for subject scores in HC.Acknowledgements
This study is supported by the National Institutes of Health (R01NS117547 and P20GM109025), a private grant from the Peter and Angela Dal Pezzo funds, a private grant from Lynn and William Weidner, a private grant from Stacie and Chuck Matthewson and the Keep Memory Alive Young Scientist Award at Cleveland Clinic Lou Ruvo Center for Brain Health. The Professional Fighters Brain Health Study is supported by Belator, UFC, the August Rapone Family Foundation, Top Rank, and Haymon Boxing.References
1. Briggs DI, Angoa-Pérez M, Kuhn DM. Prolonged Repetitive Head Trauma Induces a Singular Chronic Traumatic Encephalopathy–Like Pathology in White Matter Despite Transient Behavioral Abnormalities. Am J Pathol. 2016;186:2869–2886.
2. Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Temkin N. Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil. United States; 2009;24:439–451.
3. Bigler ED. Traumatic brain injury, neuroimaging, and neurodegeneration. Front Hum Neurosci. Frontiers Media S.A.; 2013;7:395.
4. Jordan BD. The clinical spectrum of sport-related traumatic brain injury. Nat Rev Neurol. England; 2013;9:222–230.
5. Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, Lipton ML. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am J Neuroradiol. United States; 2013;34:2064–2074.
6. Mishra VR, Zhuang X, Sreenivasan KR, et al. Multimodal MR imaging signatures of cognitive impairment in active professional fighters. Radiology. 2017;285.
7. Ng TSC, Lin AP, Koerte IK, et al. Neuroimaging in repetitive brain trauma. Alzheimers Res Ther. BioMed Central; 2014;6:10.
8. Orrison WW, Hanson EH, Alamo T, et al. Traumatic brain injury: a review and high-field MRI findings in 100 unarmed combatants using a literature-based checklist approach. J Neurotrauma. 2009;26:689–701.
9. Shin W, Mahmoud SY, Sakaie K, et al. Diffusion Measures Indicate Fight Exposure-Related Damage to Cerebral White Matter in Boxers and Mixed Martial Arts Fighters. Am J Neuroradiol. 2014;35:285–290.
10. Wintermark M, Sanelli PC, Anzai Y, Tsiouris AJ, Whitlow CT. Imaging evidence and recommendations for traumatic brain injury: advanced neuro- and neurovascular imaging techniques. AJNR Am J Neuroradiol. United States; 2015;36:E1–E11.
11. Zhang L, Heier LA, Zimmerman RD, Jordan B, Ulug AM. Diffusion anisotropy changes in the brains of professional boxers. AJNR Am J Neuroradiol. 2006;27:2000–2004.
12. Zhang L, Ravdin LD, Relkin N, et al. Increased diffusion in the brain of professional boxers: a preclinical sign of traumatic brain injury? AJNR Am J Neuroradiol. 2003;24:52–57.
13. Guye M, Bettus G, Bartolomei F, Cozzone PJ. Graph theoretical analysis of structural and functional connectivity MRI in normal and pathological brain networks. MAGMA. Germany; 2010;23:409–421.
14. Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch Clin Neuropsychol. 2006;21:623–643.
15. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. United States; 2016;125:1063–1078.
16. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. United States; 2002;15:273–289.
17. Keuken MC, Bazin P-L, Crown L, et al. Quantifying inter-individual anatomical variability in the subcortex using 7 T structural MRI. Neuroimage. United States; 2014;94:40–46.
18. Wang R, Wedeen VJ. TrackVis.org. Proc Intl Soc Mag Reson Med. 2007. p. 3720.
19. Cheng H, Wang Y, Sheng J, et al. Optimization of seed density in DTI tractography for structural networks. J Neurosci Methods. 2011/09/29. 2012;203:264–272.
20. Mishra VR, Sreenivasan KR, Zhuang X, et al. Understanding white matter structural connectivity differences between cognitively impaired and nonimpaired active professional fighters. Hum Brain Mapp. 2019;40:5108–5122.
21. Wang J, Wang X, Xia M, Liao X, Evans A, He Y. GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front Hum Neurosci. 2015;9:386.
22. Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage. United States; 2010;53:1197–1207.
23. Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. United States; 2014;92:381–397.
Figures